Monosynaptic circuit tracing in vivo through Cre-dependent targeting and complementation of modified rabies virus
- PMID: 21115815
- PMCID: PMC3003023
- DOI: 10.1073/pnas.1011756107
Monosynaptic circuit tracing in vivo through Cre-dependent targeting and complementation of modified rabies virus
Abstract
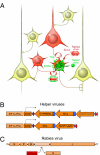
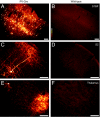
We describe a powerful system for revealing the direct monosynaptic inputs to specific cell types in Cre-expressing transgenic mice through the use of Cre-dependent helper virus and a modified rabies virus. We generated helper viruses that target gene expression to Cre-expressing cells, allowing us to control initial rabies virus infection and subsequent monosynaptic retrograde spread. Investigators can use this system to elucidate the connections onto a desired cell type in a high-throughput manner, limited only by the availability of Cre mouse lines. This method allows for identification of circuits that would be extremely tedious or impossible to study with other methods and can be used to build subcircuit maps of inputs onto many different types of cells within the same brain region. Furthermore, by expressing various transgenes from the rabies genome, this system also has the potential to allow manipulation of targeted neuronal circuits without perturbing neighboring cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

