Activation and intrinsic gamma-secretase activity of presenilin 1
- PMID: 21115843
- PMCID: PMC3003001
- DOI: 10.1073/pnas.1013246107
Activation and intrinsic gamma-secretase activity of presenilin 1
Abstract
A complex composed of presenilin (PS), nicastrin, PEN-2, and APH-1 is absolutely required for γ-secretase activity in vivo. Evidence has emerged to suggest a role for PS as the catalytic subunit of γ-secretase, but it has not been established that PS is catalytically active in the absence of associated subunits. We now report that bacterially synthesized, recombinant PS (rPS) reconstituted into liposomes exhibits γ-secretase activity. Moreover, an rPS mutant that lacks a catalytic aspartate residue neither exhibits reconstituted γ-secretase activity nor interacts with a transition-state γ-secretase inhibitor. Importantly, we demonstrate that rPS harboring mutations that cause early onset familial Alzheimer's disease (FAD) lead to elevations in the ratio of Aβ42 to Aβ40 peptides produced from a wild-type APP substrate and that rPS enhances the Aβ42/Aβ40 peptide ratio from FAD-linked mutant APP substrates, findings that are entirely consistent with the results obtained in in vivo settings. Thus, γ-secretase cleavage specificity is an inherent property of the polypeptide. Finally, we demonstrate that PEN2 is sufficient to promote the endoproteolysis of PS1 to generate the active form of γ-secretase. Thus, we conclusively establish that activated PS is catalytically competent and the bimolecular interaction of PS1 and PEN2 can convert the PS1 zymogen to an active protease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


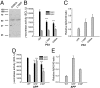

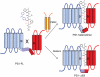
Comment in
-
And four equals one: presenilin takes the gamma-secretase role by itself.Proc Natl Acad Sci U S A. 2010 Dec 14;107(50):21236-7. doi: 10.1073/pnas.1016284108. Epub 2010 Dec 6. Proc Natl Acad Sci U S A. 2010. PMID: 21135249 Free PMC article. No abstract available.
References
-
- De Strooper B. Aph-1, Pen-2, and nicastrin with presenilin generate an active gamma-secretase complex. Neuron. 2003;38:9–12. - PubMed
-
- Edbauer D, et al. Reconstitution of gamma-secretase activity. Nat Cell Biol. 2003;5:486–488. - PubMed
-
- Thinakaran G, et al. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron. 1996;17:181–190. - PubMed
-
- Takasugi N, et al. The role of presenilin cofactors in the gamma-secretase complex. Nature. 2003;422:438–441. - PubMed
-
- Luo WJ, et al. PEN-2 and APH-1 coordinately regulate proteolytic processing of presenilin 1. J Biol Chem. 2003;278:7850–7854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

