Supramolecular self-assembled chaos: polyphenolic lignin's barrier to cost-effective lignocellulosic biofuels
- PMID: 21116223
- PMCID: PMC6259226
- DOI: 10.3390/molecules15118641
Supramolecular self-assembled chaos: polyphenolic lignin's barrier to cost-effective lignocellulosic biofuels
Abstract
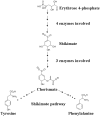
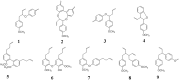
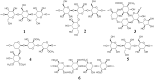
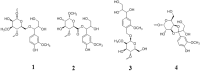
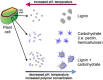
Phenylpropanoid metabolism yields a mixture of monolignols that undergo chaotic, non-enzymatic reactions such as free radical polymerization and spontaneous self-assembly in order to form the polyphenolic lignin which is a barrier to cost-effective lignocellulosic biofuels. Post-synthesis lignin integration into the plant cell wall is unclear, including how the hydrophobic lignin incorporates into the wall in an initially hydrophilic milieu. Self-assembly, self-organization and aggregation give rise to a complex, 3D network of lignin that displays randomly branched topology and fractal properties. Attempts at isolating lignin, analogous to archaeology, are instantly destructive and non-representative of in planta. Lack of plant ligninases or enzymes that hydrolyze specific bonds in lignin-carbohydrate complexes (LCCs) also frustrate a better grasp of lignin. Supramolecular self-assembly, nano-mechanical properties of lignin-lignin, lignin-polysaccharide interactions and association-dissociation kinetics affect biomass deconstruction and thereby cost-effective biofuels production.
Figures


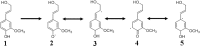

References
-
- Vacek J., Ulrichova J., Klejdus B., Simanek V. Analytical methods and strategies in the study of plant polyphenolics in clinical samples. Anal. Meth. 2010;2:604–613. doi: 10.1039/c0ay00042f. - DOI
-
- Kenrick P., Crane P.R. The origin and early evolution of plants on land. Nature. 1997;389:33–39. doi: 10.1038/37918. - DOI
-
- Ralph J., Lundquist K., Brunow G., Lu F., Kim H., Schatz P.F., Marita J.M., Hatfield R.D., Ralph S.A., Christensen J.H., Boerjan W. Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenylpropanoids. Phytochem. Rev. 2004;3:29–60. doi: 10.1023/B:PHYT.0000047809.65444.a4. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

