Preformed gentamicin spacers in two-stage revision hip arthroplasty: functional results and complications
- PMID: 21116817
- PMCID: PMC3174299
- DOI: 10.1007/s00264-010-1172-8
Preformed gentamicin spacers in two-stage revision hip arthroplasty: functional results and complications
Abstract
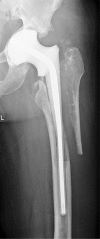
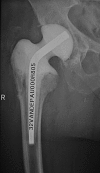
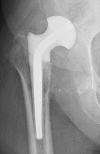
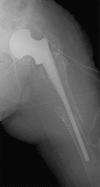
Two-stage revisions with antibiotic-loaded spacers have gained popularity for treating infected hip-joint arthroplasties. The aim of this prospective study was to assess patient functionality between stages and treatment impact on duration of hospital stay and to describe related complications. Sixty-one consecutive patients with infected hip arthroplasties underwent two-stage revision with preformed spacer implantation. Mean Harris Hip and Merle d'Aubigné scores between the two stages were 39.9 and 7.6, respectively. Forty-six patients (75.4%) were able to leave hospital between stages. Spacer dislocation occurred in 16.4%. No cases of spacer breakage were noted. Preformed cement spacers provide acceptable functional outcome between revision hip arthroplasty stages and facilitate the surgical procedure without increasing mechanical complication rates.
Figures

References
-
- Koo KH, Yang JW, Cho SH, Song HR, Park HB, Ha YC, Chang JD, Kin SY, Kim YH. Impregnation of vancomycin, gentamycin, and cefotaxime in the cement spacer for two-stage cementless reconstruction in infected total hip arthroplasty. J Arthroplasty. 2001;16:882–892. doi: 10.1054/arth.2001.24444. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

