A mechanism by which binding of the broadly neutralizing antibody b12 unfolds the inner domain α1 helix in an engineered HIV-1 gp120
- PMID: 21117239
- PMCID: PMC3059550
- DOI: 10.1002/prot.22901
A mechanism by which binding of the broadly neutralizing antibody b12 unfolds the inner domain α1 helix in an engineered HIV-1 gp120
Abstract
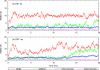
Using all-atom simulations, we examine the role of the I109C/Q428C disulfide "stitch" in altering the conformational distribution of engineered HIV-1 gp120 core relevant for binding of the broadly neutralizing recombinant antibody b12. In particular, we propose that the I109C/Q428C stitch results in a conformational distribution favoring an unfolded inner-domain α1-helix upon binding of b12. Using targeted molecular dynamics, we show that folded α1 in the b12-bound conformation of gp120 is stable both with and without the stitch, but that with folded α1, the stitch requires an orientation of the β20/β21 sheet that is sterically incompatible with b12 binding. Forcing β20/β21 into the orientation displayed by the b12-bound conformation after folding α1 with the stitch intact results in partial unfolding of α1, whereas without the stitch, β20/β21 reorientation does not affect the conformation of α1. These findings collectively support the hypothesis that the disulfide stitch shifts the conformational distribution of α1 to the unfolded state, meaning an unfolded α1 is not a strict requirement of the b12-bound conformational ensemble of gp120's lacking the I109C/Q428C stitch.
© 2010 Wiley-Liss, Inc.
Figures






References
-
- Poignard P, Saphire E, Parren P, Burton D. Gp120: Biologic aspects of structural features. Annu Rev Immunol. 2001;19:253–274. - PubMed
-
- Phogat S, Wyatt R. Rational modifications of hiv-1 envelope glycoproteins for immunogen design. Curr Pharm Design. 2007;13:213–227. - PubMed
-
- Wyatt R, Sodroski J. The hiv-1 envelope glycoproteins: Fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

