Intracellular delivery of bioactive molecules using light-addressable nanocapsules
- PMID: 21117640
- PMCID: PMC3075813
- DOI: 10.1021/nn102345f
Intracellular delivery of bioactive molecules using light-addressable nanocapsules
Abstract
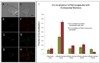
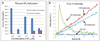
This paper describes a method by which molecules that are impermeable to cells are encapsulated in dye-sensitized lipid nanocapsules for delivery into cells via endocytosis. Once inside the cells, the molecules are released from the lipid nanocapsules into the cytoplasm with a single nanosecond pulse from a laser in the far red (645 nm). We demonstrate this method with the intracellular release of the second messenger IP(3) in CHO-M1 cells and report that calcium responses from the cells changed from a sustained increase to a transient spike when the average number of IP(3) released is decreased below 50 molecules per nanocapsule. We also demonstrate the delivery of a 23 kDa O(6)-alkylguanine-DNA alkyltransferase (AGT) fusion protein into Ba/F3 cells to inhibit a key player BCR-ABL in the apoptotic pathway. We show that an average of ∼8 molecules of the inhibitor is sufficient to induce apoptosis in the majority of Ba/F3 cells.
Figures



References
-
- Gug S, Charon S, Specht A, Alarcon K, Ogden D, Zietz B, Leonard J, Haacke S, Bolze F, Nicoud JF, et al. M. Photolabile Glutamate Protecting Group with High One- and Two-Photon Uncaging Efficiencies. ChemBioChem. 2008;9:1303–1307. - PubMed
-
- Warther D, Bolze F, Leonard J, Gug S, Specht A, Puliti D, Sun XH, Kessler P, Lutz Y, Vonesch JL, et al. Live-cell One- and Two-Photon Uncaging of a Far-Red Emitting Acridinone Fluorophore. J. Am. Chem. Soc. 2010;132:2585–2590. - PubMed
-
- Nikolenko V, Poskanzer KE, Yuste R. Two-Photon Photostimulation and Imaging of Neural Circuits. Nat. Methods. 2007;4:943–950. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

