Design of the Resistance and Endurance exercise After ChemoTherapy (REACT) study: a randomized controlled trial to evaluate the effectiveness and cost-effectiveness of exercise interventions after chemotherapy on physical fitness and fatigue
- PMID: 21118564
- PMCID: PMC3009679
- DOI: 10.1186/1471-2407-10-658
Design of the Resistance and Endurance exercise After ChemoTherapy (REACT) study: a randomized controlled trial to evaluate the effectiveness and cost-effectiveness of exercise interventions after chemotherapy on physical fitness and fatigue
Abstract
Background: Preliminary studies suggest that physical exercise interventions can improve physical fitness, fatigue and quality of life in cancer patients after completion of chemotherapy. Additional research is needed to rigorously test the effects of exercise programmes among cancer patients and to determine optimal training intensity accordingly. The present paper presents the design of a randomized controlled trial evaluating the effectiveness and cost-effectiveness of a high intensity exercise programme compared to a low-to-moderate intensity exercise programme and a waiting list control group on physical fitness and fatigue as primary outcomes.
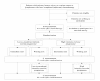
Methods: After baseline measurements, cancer patients who completed chemotherapy are randomly assigned to either a 12-week high intensity exercise programme or a low-to-moderate intensity exercise programme. Next, patients from both groups are randomly assigned to immediate training or a waiting list (i.e. waiting list control group). After 12 weeks, patients of the waiting list control group start with the exercise programme they have been allocated to.Both interventions consist of equal bouts of resistance and endurance interval exercises with the same frequency and duration, but differ in training intensity. Additionally, patients of both exercise programmes are counselled to improve compliance and achieve and maintain an active lifestyle, tailored to their individual preferences and capabilities.Measurements will be performed at baseline (t = 0), 12 weeks after randomization (t = 1), and 64 weeks after randomization (t = 2). The primary outcome measures are cardiorespiratory fitness and muscle strength assessed by means of objective performance indicators, and self-reported fatigue. Secondary outcome measures include health-related quality of life, self-reported physical activity, daily functioning, body composition, mood and sleep disturbances, and return to work. In addition, compliance and satisfaction with the interventions will be evaluated. Potential moderation by pre- and post-illness lifestyle, health and exercise-related attitudes, beliefs and motivation will also be assessed. Finally, the cost-effectiveness of both exercise interventions will be evaluated.
Discussion: This randomized controlled trial will be a rigorous test of effects of exercise programmes for cancer patients after chemotherapy, aiming to contribute to evidence-based practice in cancer rehabilitation programmes.
Trial registration: This study is registered at the Netherlands Trial Register (NTR2153).
Figures
References
-
- iKCnet - Kankerregistratie. http://ikcnet.nl
-
- Pihkala J, Happonen JM, Virtanen K, Sovijarvi A, Siimes MA, Pesonen E. et al.Cardiopulmonary evaluation of exercise tolerance after chest irradiation and anticancer chemotherapy in children and adolescents. Pediatrics. 1995;95:722–726. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical