Estrogen aggravates inflammation in Pseudomonas aeruginosa pneumonia in cystic fibrosis mice
- PMID: 21118573
- PMCID: PMC3006363
- DOI: 10.1186/1465-9921-11-166
Estrogen aggravates inflammation in Pseudomonas aeruginosa pneumonia in cystic fibrosis mice
Abstract
Background: Among patients with cystic fibrosis (CF), females have worse pulmonary function and survival than males, primarily due to chronic lung inflammation and infection with Pseudomonas aeruginosa (P. aeruginosa). A role for gender hormones in the causation of the CF "gender gap" has been proposed. The female gender hormone 17β-estradiol (E2) plays a complex immunomodulatory role in humans and in animal models of disease, suppressing inflammation in some situations while enhancing it in others. Helper T-cells were long thought to belong exclusively to either T helper type 1 (Th1) or type 2 (Th2) lineages. However, a distinct lineage named Th17 is now recognized that is induced by interleukin (IL)-23 to produce IL-17 and other pro-inflammatory Th17 effector molecules. Recent evidence suggests a central role for the IL-23/IL-17 pathway in the pathogenesis of CF lung inflammation. We used a mouse model to test the hypothesis that E2 aggravates the CF lung inflammation that occurs in response to airway infection with P. aeruginosa by a Th17-mediated mechanism.
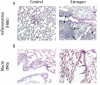
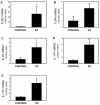
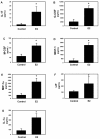
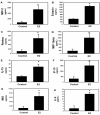
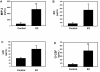
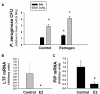
Results: Exogenous E2 caused adult male CF mice with pneumonia due to a mucoid CF clinical isolate, the P. aeruginosa strain PA508 (PA508), to develop more severe manifestations of inflammation in both lung tissue and in bronchial alveolar lavage (BAL) fluid, with increased total white blood cell counts and differential and absolute cell counts of polymorphonuclear leukocytes (neutrophils). Inflammatory infiltrates and mucin production were increased on histology. Increased lung tissue mRNA levels for IL-23 and IL-17 were accompanied by elevated protein levels of Th17-associated pro-inflammatory mediators in BAL fluid. The burden of PA508 bacteria was increased in lung tissue homogenate and in BAL fluid, and there was a virtual elimination in lung tissue of mRNA for lactoferrin, an antimicrobial peptide active against P. aeruginosa in vitro.
Conclusions: Our data show that E2 increases the severity of PA508 pneumonia in adult CF male mice, and suggest two potential mechanisms: enhancement of Th17-regulated inflammation and suppression of innate antibacterial defences. Although this animal model does not recapitulate all aspects of human CF lung disease, our present findings argue for further investigation of the effects of E2 on inflammation and infection with P. aeruginosa in the CF lung.
Figures


References
-
- Corey M, Farewell V. Determinants of mortality from cystic fibrosis in Canada, 1970-1989. Am J Epidemiol. 1996;143:1007–1017. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

