Midbrain dopaminergic axons are guided longitudinally through the diencephalon by Slit/Robo signals
- PMID: 21118670
- PMCID: PMC3021181
- DOI: 10.1016/j.mcn.2010.11.003
Midbrain dopaminergic axons are guided longitudinally through the diencephalon by Slit/Robo signals
Abstract
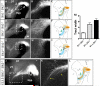
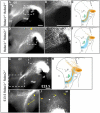
Dopaminergic neurons from the ventral mesencephalon/diencephalon (mesodiencephalon) form vital pathways constituting the majority of the brain's dopamine systems. Mesodiencephalic dopaminergic (mdDA) neurons extend longitudinal projections anteriorly through the diencephalon, ascending toward forebrain targets. The mechanisms by which mdDA axons initially navigate through the diencephalon are poorly understood. Recently the Slit family of secreted axon guidance proteins, and their Robo receptors, have been identified as important guides for descending longitudinal axons. To test the potential roles of Slit/Robo guidance in ascending trajectories, we examined tyrosine hydroxylase-positive (TH+) projections from mdDA neurons in mutant mouse embryos. We found that mdDA axons grow out of and parallel to Slit-positive ventral regions within the diencephalon, and that subsets of the mdDA axons likely express Robo1 and possibly also Robo2. Slit2 was able to directly inhibit TH axon outgrowth in explant co-culture assays. The mdDA axons made significant pathfinding errors in Slit1/2 and Robo1/2 knockout mice, including spreading out in the diencephalon to form a wider tract. The wider tract resulted from a combination of invasion of the ventral midline, consistent with Slit repulsion, but also axons wandering dorsally, away from the ventral midline. Aberrant dorsal trajectories were prominent in Robo1 and Robo1/2 knockout mice, suggesting that an aspect of Robo receptor function is Slit-independent. These results indicate that Slit/Robo signaling is critical during the initial establishment of dopaminergic pathways, with roles in the dorsoventral positioning and precise pathfinding of these ascending longitudinal axons.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures



References
-
- Bagri A, Marin O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, Tessier-Lavigne M. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002;33:233–248. - PubMed
-
- Devine CA, Key B. Robo-Slit interactions regulate longitudinal axon pathfinding in the embryonic vertebrate brain. Dev Biol. 2008;313:371–383. - PubMed
-
- Dickson BJ, Gilestro GF. Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu Rev Cell Dev Biol. 2006;22:651–675. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

