Patterns of baseline autonomic nerve activity and the development of pacing-induced sustained atrial fibrillation
- PMID: 21118728
- PMCID: PMC3065927
- DOI: 10.1016/j.hrthm.2010.11.040
Patterns of baseline autonomic nerve activity and the development of pacing-induced sustained atrial fibrillation
Abstract
Background: Whether autonomic nerve activity is important in the development of pacing-induced sustained atrial fibrillation (AF) is unclear.
Objective: The purpose of this study was to test the hypothesis that patterns of baseline autonomic nerve activity are important in the development of pacing-induced sustained AF.
Methods: Radiotransmitters were implanted in 12 ambulatory dogs to record left stellate ganglion nerve activity (SGNA) and vagal nerve activity (VNA). Sustained (>48 hours) AF was induced with intermittent rapid atrial pacing.
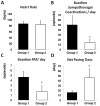
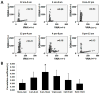
Results: At baseline (before pacing), 1-minute integrated nerve activity between SGNA and VNA demonstrated either a single linear relationship with excellent correlation (group 1, N = 3, r = 0.816 ± 0.105) or nonlinear relationships with poor correlation (group 2, N = 9, r = 0.316 ± 0.162, P <.05 vs group 1). Group 1 dogs had higher VNA (97.0 ± 11.5 mV-s) compared to group 2 (33.4 ± 21.7 mV-s, P <.001). Group 1 dogs had more frequent sympathovagal co-activation episodes than did group 2 (50 ± 19 per day vs 15 ± 6 per day, P <.05) and more paroxysmal atrial tachycardia (PAT; 5 ± 1 per day vs 2 ± 1 per day, P <.05) at baseline. Sustained AF occurred after 16 ± 4 days (range 13-20 days) of pacing in group 1 and after 46 ± 18 days (range 23-72 days) of pacing in group 2 (P <.05). In the week before development of sustained AF, VNA of group 2 dogs was significantly increased compared to baseline (P <.05).
Conclusion: Ambulatory dogs with good linear sympathovagal correlation and higher vagal tone at baseline have more PAT episodes at baseline and faster induction of sustained AF by rapid pacing. Rapid atrial pacing increased the VNA of the remaining dogs before induction of sustained AF.
Copyright © 2011 Heart Rhythm Society. Published by Elsevier Inc. All rights reserved.
Figures




Comment in
-
The cardiac neuronal hierarchy and susceptibility to arrhythmias.Heart Rhythm. 2011 Apr;8(4):590-1. doi: 10.1016/j.hrthm.2010.12.019. Epub 2010 Dec 16. Heart Rhythm. 2011. PMID: 21167959 Free PMC article. No abstract available.
References
-
- Kerr CR, Humphries KH, Talajic M, et al. Progression to chronic atrial fibrillation after the initial diagnosis of paroxysmal atrial fibrillation: results from the Canadian Registry of Atrial Fibrillation. Am Heart J. 2005;149:489–496. - PubMed
-
- Jahangir A, Lee V, Friedman PA, et al. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: a 30-year follow-up study. Circulation. 2007;115:3050–3056. - PubMed
-
- Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

