Prostate segmentation in HIFU therapy
- PMID: 21118767
- PMCID: PMC3095593
- DOI: 10.1109/TMI.2010.2095465
Prostate segmentation in HIFU therapy
Abstract
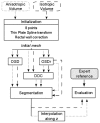
Prostate segmentation in 3-D transrectal ultrasound images is an important step in the definition of the intra-operative planning of high intensity focused ultrasound (HIFU) therapy. This paper presents two main approaches for the semi-automatic methods based on discrete dynamic contour and optimal surface detection. They operate in 3-D and require a minimal user interaction. They are considered both alone or sequentially combined, with and without postregularization, and applied on anisotropic and isotropic volumes. Their performance, using different metrics, has been evaluated on a set of 28 3-D images by comparison with two expert delineations. For the most efficient algorithm, the symmetric average surface distance was found to be 0.77 mm.
Figures









References
-
- Cancer facts and figures. Am Cancer Soc. 2010. [Online]. Available: http://www.cancer.org.
-
- European Cancer Observatory [Online] Available: http://eu-cancer.iarc.fr.
-
- Damianou CA, Hynynen K, Fan X. Evaluation of accuracy of a theoretical model for predicting the necrosed tissue volume during focused ultrasound surgery. IEEE Trans Ultrason Ferroelectr Freq Control. 1995 Mar;42(2):182–187.
-
- Curiel L, Chavrier F, Gignoux B, Pichardo S, Chesnais S, Chapelon JY. Experimental evaluation of lesion prediction modelling in the presence of cavitation bubbles: Intended for high-intensity focused ultrasound prostate treatment. Med Biol Eng Comput. 2004 Jan;42(1):44–54. - PubMed
-
- Aarnink RG, Giesen RJ, Huynen AL, de la Rosette JJ, Debruyne FM, Wijkstra H. A practical clinical method for contour determination in ultrasonographic prostate images. Ultrasound Med Biol. 1994;20(8):705–717. - PubMed

