Non-specific effects of standard measles vaccine at 4.5 and 9 months of age on childhood mortality: randomised controlled trial
- PMID: 21118875
- PMCID: PMC2994348
- DOI: 10.1136/bmj.c6495
Non-specific effects of standard measles vaccine at 4.5 and 9 months of age on childhood mortality: randomised controlled trial
Abstract
Objective: To examine in a randomised trial whether a 25% difference in mortality exists between 4.5 months and 3 years of age for children given two standard doses of Edmonston-Zagreb measles vaccines at 4.5 and 9 months of age compared with those given one dose of measles vaccine at 9 months of age (current policy).
Design: Randomised controlled trial.
Setting: The Bandim Health Project, Guinea-Bissau, which maintains a health and demographic surveillance system in an urban area.
Participants: 6648 children aged 4.5 months of age who had received three doses of diphtheria-tetanus-pertussis vaccine at least four weeks before enrolment. A large proportion of the children (80%) had previously taken part in randomised trials of neonatal vitamin A supplementation.
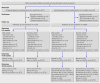
Intervention: Children were randomised to receive Edmonston-Zagreb measles vaccine at 4.5 and 9 months of age (group A), no vaccine at 4.5 months and Edmonston-Zagreb measles vaccine at 9 months of age (group B), or no vaccine at 4.5 months and Schwarz measles vaccine at 9 months of age (group C). Main outcome measure Mortality rate ratio between 4.5 and 36 months of age for group A compared with groups B and C. Secondary outcomes tested the hypothesis that the beneficial effect was stronger in the 4.5 to 9 months age group, in girls, and in the dry season, but the study was not powered to test whether effects differed significantly between subgroups.
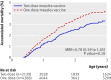
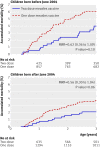
Results: In the intention to treat analysis of mortality between 4.5 and 36 months of age the mortality rate ratio of children who received two doses of Edmonston-Zagreb vaccine at 4.5 and 9 months of age compared with those who received a single dose of Edmonston-Zagreb vaccine or Schwarz vaccine at 9 months of age was 0.78 (95% confidence interval 0.59 to 1.05). In the analyses of secondary outcomes, the intention to treat mortality rate ratio was 0.67 (0.38 to 1.19) between 4.5 and 9 months and 0.83 (0.83 to 1.16) between 9 and 36 months of age. The effect on mortality between 4.5 and 36 months of age was significant for girls (intention to treat mortality rate ratio 0.64 (0.42 to 0.98)), although this was not significantly different from the effect in boys (0.95 (0.64 to 1.42)) (interaction test, P=0.18). The effect did not differ between the dry season and the rainy season. As neonatal vitamin A supplementation is not WHO policy, the analyses were done separately for the 3402 children who did not receive neonatal vitamin A. In these children, the two dose Edmonston-Zagreb measles vaccine schedule was associated with a significantly lower mortality between 4.5 and 36 months of age (intention to treat mortality rate ratio 0.59 (0.39 to 0.89)). The effect was again significant for girls but not statistically significant from the effect in boys. When measles cases were censored, the intention to treat mortality rate ratio was 0.65 (0.43 to 0.99).
Conclusions: Although the overall effect did not reach statistical significance, the results may indicate that a two dose schedule with Edmonston-Zagreb measles vaccine given at 4.5 and 9 months of age has beneficial non-specific effects on children's survival, particularly for girls and for children who have not received neonatal vitamin A. This should be tested in future studies in different locations.
Trial registration: Clinical trials NCT00168558.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
Figures
References
-
- Kasongo Project Team. Influence of measles vaccination on survival pattern of 7-35-month-old children in Kasongo, Zaire. Lancet 1981;1:764-7. - PubMed
-
- Aaby P, Bukh J, Lisse IM, Smits AJ. Measles vaccination and child mortality. Lancet 1981;ii:93. - PubMed
-
- Aaby P, Bukh J, Lisse IM, Smits AJ. Measles vaccination and reduction in child mortality: a community study from Guinea-Bissau. J Infect 1984;8:13-21. - PubMed
-
- Desgrées du Loû A, Pison G, Aaby P. The role of immunizations in the recent decline in childhood mortality and the changes in the female/male mortality ratio in rural Senegal. Am J Epidemiol 1995;142:643-52. - PubMed
-
- Aaby P, Samb B, Simondon F, Knudsen K, Coll Seck AM, Bennett J, et al. Divergent mortality for male and female recipients of low-titre and high-titre measles vaccines in rural Senegal. Am J Epidemiol 1993;138:746-55. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
