miRNA-132 orchestrates chromatin remodeling and translational control of the circadian clock
- PMID: 21118894
- PMCID: PMC3115667
- DOI: 10.1093/hmg/ddq519
miRNA-132 orchestrates chromatin remodeling and translational control of the circadian clock
Abstract
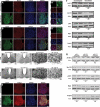
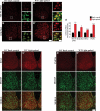
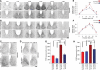
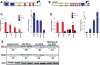
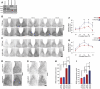
Mammalian circadian rhythms are synchronized to the external time by daily resetting of the suprachiasmatic nucleus (SCN) in response to light. As the master circadian pacemaker, the SCN coordinates the timing of diverse cellular oscillators in multiple tissues. Aberrant regulation of clock timing is linked to numerous human conditions, including cancer, cardiovascular disease, obesity, various neurological disorders and the hereditary disorder familial advanced sleep phase syndrome. Additionally, mechanisms that underlie clock resetting factor into the sleep and physiological disturbances experienced by night-shift workers and travelers with jet lag. The Ca(2+)/cAMP response element-binding protein-regulated microRNA, miR-132, is induced by light within the SCN and attenuates its capacity to reset, or entrain, the clock. However, the specific targets that are regulated by miR-132 and underlie its effects on clock entrainment remained elusive until now. Here, we show that genes involved in chromatin remodeling (Mecp2, Ep300, Jarid1a) and translational control (Btg2, Paip2a) are direct targets of miR-132 in the mouse SCN. Coordinated regulation of these targets underlies miR-132-dependent modulation of Period gene expression and clock entrainment: the mPer1 and mPer2 promoters are bound to and transcriptionally activated by MeCP2, whereas PAIP2A and BTG2 suppress the translation of the PERIOD proteins by enhancing mRNA decay. We propose that miR-132 is selectively enriched for chromatin- and translation-associated target genes and is an orchestrator of chromatin remodeling and protein translation within the SCN clock, thereby fine-tuning clock entrainment. These findings will further our understanding of mechanisms governing clock entrainment and its involvement in human diseases.
Figures




References
-
- Reppert S.M., Weaver D.R. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi:10.1038/nature00965. - DOI - PubMed
-
- Toh K.L., Jones C.R., He Y., Eide E.J., Hinz W.A., Virshup D.M., Ptácek L.J., Fu Y.H. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291:1040–1043. doi:10.1126/science.1057499. - DOI - PubMed
-
- Xu Y., Padiath Q.S., Shapiro R.E., Jones C.R., Wu S.C., Saigoh N., Saigoh K., Ptácek L.J., Fu Y.H. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature. 2005;434:640–644. doi:10.1038/nature03453. - DOI - PubMed
-
- Ebisawa T., Uchiyama M., Kajimura N., Mishima K., Kamei Y., Katoh M., Watanabe T., Sekimoto M., Shibui K., Kim K., et al. Association of structural polymorphisms in the human period3 gene with delayed sleep phase syndrome. EMBO Rep. 2001;2:342–346. doi:10.1093/embo-reports/kve070. - DOI - PMC - PubMed
-
- Panda S., Antoch M.P., Miller B.H., Su A.I., Schook A.B., Straume M., Schultz P.G., Kay S.A., Takahashi J.S., Hogenesch J.B. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–220. doi:10.1016/S0092-8674(02)00722-5. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

