The kinesin KIF9 and reggie/flotillin proteins regulate matrix degradation by macrophage podosomes
- PMID: 21119006
- PMCID: PMC3020916
- DOI: 10.1091/mbc.E10-05-0394
The kinesin KIF9 and reggie/flotillin proteins regulate matrix degradation by macrophage podosomes
Abstract
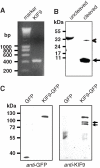
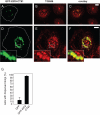
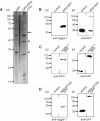
Podosomes are actin-based matrix contacts in a variety of cell types, most notably monocytic cells, and are characterized by their ability to lyse extracellular matrix material. Besides their dependence on actin regulation, podosomes are also influenced by microtubules and microtubule-dependent transport processes. Here we describe a novel role for KIF9, a previously little-characterized member of the kinesin motor family, in the regulation of podosomes in primary human macrophages. We find that small interfering RNA (siRNA)/short-hairpin RNA-induced knockdown of KIF9 significantly affects both numbers and matrix degradation of podosomes. Overexpression and microinjection experiments reveal that the unique C-terminal region of KIF9 is crucial for these effects, presumably through binding of specific interactors. Indeed, we further identify reggie-1/flotillin-2, a signaling mediator between intracellular vesicles and the cell periphery, as an interactor of the KIF9 C-terminus. Reggie-1 dynamically colocalizes with KIF9 in living cells, and, consistent with KIF9-mediated effects, siRNA-induced knockdown of reggies/flotillins significantly impairs matrix degradation by podosomes. In sum, we identify the kinesin KIF9 and reggie/flotillin proteins as novel regulators of macrophage podosomes and show that their interaction is critical for the matrix-degrading ability of these structures.
Figures







Similar articles
-
Microtubule acetylation regulates dynamics of KIF1C-powered vesicles and contact of microtubule plus ends with podosomes.Eur J Cell Biol. 2014 Oct;93(10-12):424-37. doi: 10.1016/j.ejcb.2014.07.006. Epub 2014 Aug 2. Eur J Cell Biol. 2014. PMID: 25151635
-
Trafficking of the microdomain scaffolding protein reggie-1/flotillin-2.Eur J Cell Biol. 2008 Apr;87(4):211-26. doi: 10.1016/j.ejcb.2007.12.001. Epub 2008 Jan 30. Eur J Cell Biol. 2008. PMID: 18237819
-
The GTPase Gem and its partner Kif9 are required for chromosome alignment, spindle length control, and mitotic progression.FASEB J. 2012 Dec;26(12):5025-34. doi: 10.1096/fj.12-209460. Epub 2012 Sep 10. FASEB J. 2012. PMID: 22964304
-
The matrix corroded: podosomes and invadopodia in extracellular matrix degradation.Trends Cell Biol. 2007 Mar;17(3):107-17. doi: 10.1016/j.tcb.2007.01.002. Epub 2007 Feb 1. Trends Cell Biol. 2007. PMID: 17275303 Review.
-
Degrading devices: invadosomes in proteolytic cell invasion.Annu Rev Cell Dev Biol. 2011;27:185-211. doi: 10.1146/annurev-cellbio-092910-154216. Epub 2011 Jul 21. Annu Rev Cell Dev Biol. 2011. PMID: 21801014 Review.
Cited by
-
Regulation of cell migration by dynamic microtubules.Semin Cell Dev Biol. 2011 Dec;22(9):968-74. doi: 10.1016/j.semcdb.2011.09.017. Epub 2011 Oct 4. Semin Cell Dev Biol. 2011. PMID: 22001384 Free PMC article. Review.
-
Supervillin couples myosin-dependent contractility to podosomes and enables their turnover.J Cell Sci. 2012 May 1;125(Pt 9):2300-14. doi: 10.1242/jcs.100032. Epub 2012 Feb 17. J Cell Sci. 2012. PMID: 22344260 Free PMC article.
-
Flotillin-1 Interacts With and Sustains the Surface Levels of TRPV2 Channel.Front Cell Dev Biol. 2021 Feb 9;9:634160. doi: 10.3389/fcell.2021.634160. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33634132 Free PMC article.
-
Nucleobindin-1 regulates ECM degradation by promoting intra-Golgi trafficking of MMPs.J Cell Biol. 2020 Aug 3;219(8):e201907058. doi: 10.1083/jcb.201907058. J Cell Biol. 2020. PMID: 32479594 Free PMC article.
-
Podosomes in space: macrophage migration and matrix degradation in 2D and 3D settings.Cell Adh Migr. 2014;8(3):179-91. doi: 10.4161/cam.28116. Cell Adh Migr. 2014. PMID: 24713854 Free PMC article. Review.
References
-
- Babuke T, Tikkanen R. Dissecting the molecular function of reggie/flotillin proteins. Eur J Cell Biol. 2007;86:525–532. - PubMed
-
- Buccione R, Caldieri G, Ayala I. Invadopodia: specialized tumor cell structures for the focal degradation of the extracellular matrix. Cancer Metastasis Rev. 2009;28:137–149. - PubMed
-
- Caldieri G, Buccione R. Aiming for invadopodia: organizing polarized delivery at sites of invasion. Trends Cell Biol. 2010;20:64–70. - PubMed
-
- Chen Proteases associated with invadopodia, and their role in degradation of extracellular matrix. Enzyme Protein. 1996;49:59–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

