Meiotic progression in Arabidopsis is governed by complex regulatory interactions between SMG7, TDM1, and the meiosis I-specific cyclin TAM
- PMID: 21119056
- PMCID: PMC3015126
- DOI: 10.1105/tpc.110.078378
Meiotic progression in Arabidopsis is governed by complex regulatory interactions between SMG7, TDM1, and the meiosis I-specific cyclin TAM
Abstract
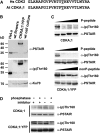
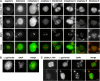
Meiosis is a modified cell division that produces four haploid nuclei from a single diploid cell in two rounds of chromosome segregation. Here, we analyze the role of Arabidopsis thaliana SUPPRESSOR WITH MORPHOGENETIC EFFECTS ON GENITALIA7 (SMG7), THREE DIVISION MUTANT1 (TDM1), and TARDY ASYNCHRONOUS MEIOSIS (TAM) in meiotic progression. SMG7 is a conserved nonsense-mediated mRNA decay factor that is also, in Arabidopsis, essential for completion of meiosis. Examination of activating CYCLIN DEPENDENT KINASE A;1 phosophorylation at Thr-161 suggests that the meiotic arrest observed in smg7 mutants is likely caused by a failure to downregulate cyclin-dependent kinase (CDK) activity at the end of the second meiotic division. Genetic analysis indicates that SMG7 and TDM1 act in the same pathway to facilitate exit from meiosis. We further demonstrate that the cyclin TAM is specifically expressed in meiosis I and has both stimulatory and inhibitory effects on progression to meiosis II. TAM knockouts skip the second meiotic division producing unreduced gametes, but inactivation of SMG7 or TDM1 alleviates TAM's requirement for entry into meiosis II. We propose a model that meiotic progression in Arabidopsis pollen mother cells is driven by a yet to be identified cyclin-CDK activity that is modulated by regulatory interactions between TDM1, SMG7, and TAM.
Figures






References
-
- Alexander M.P. (1969). Differential staining of aborted and nonaborted pollen. Stain Technol. 44: 117–122 - PubMed
-
- Averbeck N., Sunder S., Sample N., Wise J.A., Leatherwood J. (2005). Negative control contributes to an extensive program of meiotic splicing in fission yeast. Mol. Cell 18: 491–498 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

