Identification of new carbohydrate and membrane protein antigens in cardiac xenotransplantation
- PMID: 21119562
- PMCID: PMC10022691
- DOI: 10.1097/TP.0b013e318203c27d
Identification of new carbohydrate and membrane protein antigens in cardiac xenotransplantation
Abstract
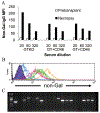
Background: α1,3-Galactosyltransferase gene knockout (GTKO) pigs reduced the significance of antibody to galactose alpha 1,3-galactose (Gal) antigens but did not eliminate delayed xenograft rejection (DXR). We hypothesize that DXR of GTKO organs results from an antibody response to a limited number of non-Gal endothelial cell (EC) membrane antigens. In this study, we screened a retrovirus expression library to identify EC membrane antigens detected after cardiac xenotransplantation.
Methods: Expression libraries were made from GT:CD46 and GTKO porcine aortic ECs. Viral stocks were used to infect human embryonic kidney cells (HEK) that were selected by flow cytometry for IgG binding from sensitized cardiac heterotopic xenograft recipients. After three to seven rounds of selection, individual clones were assessed for non-Gal IgG binding. The porcine complementary DNA was recovered by polymerase chain reaction amplification, sequenced, and identified by homology comparisons.
Results: A total of 199 and 317 clones were analyzed from GT:CD46 and GTKO porcine aortic EC complementary DNA libraries, respectively. Sequence analysis identified porcine CD9, CD46, CD59, and the EC protein C receptor. We also identified porcine annexin A2 and a glycosyltransferase with homology to the human β1,4 N-acetylgalactosaminyl transferase 2 gene.
Conclusion: The identified proteins include key EC functions and suggest that non-Gal antibody responses may compromise EC functions and thereby contribute to DXR. Recovery of the porcine β1,4 N-acetylgalactosaminyl transferase 2 suggests that an antibody response to a SD-like carbohydrate may represent a new carbohydrate moiety involved in xenotransplantation. The identification of these porcine gene products may lead to further donor modification to enhance resistance to DXR and further reduce the level of xenograft antigenicity.
Conflict of interest statement
G.W.B. and C.G.A.M. are the inventors of technology related to xenotransplantation that has been licensed by the Mayo Clinic to a commercial entity. The other authors declare no conflict of interest.
Figures

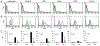
References
-
- Platt JL, Lin SS, McGregor CG. Acute vascular rejection. Xenotransplantation 1998; 5: 169. - PubMed
-
- Cowan PJ. Coagulation and the xenograft endothelium. Xenotransplantation 2007; 14: 7. - PubMed
-
- Byrne GW, Schwarz A, Fesi JR, et al. Evaluation of different alpha-galactosyl glycoconjugates for use in xenotransplantation. Bioconjug Chem 2002; 13: 571. - PubMed
-
- Lai L, Kolber-Simonds D, Park KW, et al. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 2002; 295: 1089. - PubMed
-
- Lam TT, Paniagua R, Shivaram G, et al. Anti-non-Gal porcine endothelial cell antibodies in acute humoral xenograft rejection of hDAF-transgenic porcine hearts in cynomolgus monkeys. Xenotransplantation 2004; 11: 531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

