Ligand-independent activation of c-Met by fibronectin and α(5)β(1)-integrin regulates ovarian cancer invasion and metastasis
- PMID: 21119598
- PMCID: PMC3069218
- DOI: 10.1038/onc.2010.532
Ligand-independent activation of c-Met by fibronectin and α(5)β(1)-integrin regulates ovarian cancer invasion and metastasis
Abstract
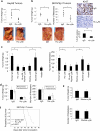
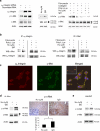
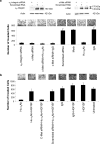
The role of the fibronectin receptor, α(5)β(1)-integrin, as an adhesion receptor and in angiogenesis is well established. However, its role in cancer cell invasion and metastasis is less clear. We describe a novel mechanism by which fibronectin regulates ovarian cancer cell signaling and promotes metastasis. Fibronectin binding to α(5)β(1)-integrin led to a direct association of α(5)-integrin with the receptor tyrosine kinase, c-Met, activating it in a hepatocyte growth factor/scatter factor (HGF/SF) independent manner. Subsequently, c-Met associated with Src, and activated Src and focal adhesion kinase (FAK). Inhibition of α(5)β(1)-integrin decreased the phosphorylation of c-Met, FAK and Src, both in vitro and in vivo. Independent activation of c-Met by its native ligand, HGF/SF, or overexpression of a constitutively active FAK in HeyA8 cells could overcome the effect of α(5)β(1)-integrin inhibition on tumor cell invasion, indicating that α(5)β(1)-integrin is upstream of c-Met, Src and FAK. Inhibition of α(5)β(1)-integrin on cancer cells in two xenograft models of ovarian cancer metastasis resulted in a significant decrease of tumor burden, which was independent of the effect of α(5)β(1)-integrin on angiogenesis. These data suggest that fibronectin promotes ovarian cancer invasion and metastasis through an α(5)β(1)-integrin/c-Met/FAK/Src-dependent signaling pathway, transducing signals through c-Met in an HGF/SF-independent manner.
Figures



Comment in
-
Met and the microenvironment: new insights for ovarian cancer metastasis.Cell Adh Migr. 2011 May-Jun;5(3):209-10. doi: 10.4161/cam.5.3.15258. Epub 2011 May 1. Cell Adh Migr. 2011. PMID: 21436615 Free PMC article.
References
-
- Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. - PubMed
-
- Brader KR, Wolf JK, Hung M-C, Yu D, Crispens MA, van Golen KL, et al. Adenovirus E1A expression enhances the sensitivity of an ovarian cancer cell line to multiple cytotoxic agents through an apoptotic mechanism. Clin Cancer Res. 1997;3:2017–2024. - PubMed
-
- Caswell PT, Spence HJ, Parsons M, White DP, Clark K, Cheng KW, et al. Rab25 associates with α5β1 integrin to promote invasive migration in 3D microenvironments. Developmental Cell. 2007;13:496–510. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

