Long-term amelioration of feline Mucopolysaccharidosis VI after AAV-mediated liver gene transfer
- PMID: 21119624
- PMCID: PMC3048181
- DOI: 10.1038/mt.2010.257
Long-term amelioration of feline Mucopolysaccharidosis VI after AAV-mediated liver gene transfer
Abstract
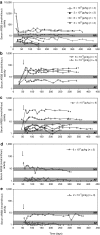
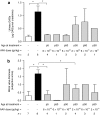
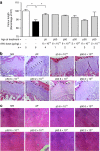
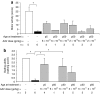
Mucopolysaccharidosis VI (MPS VI) is caused by deficient arylsulfatase B (ARSB) activity resulting in lysosomal storage of glycosaminoglycans (GAGs). MPS VI is characterized by dysostosis multiplex, organomegaly, corneal clouding, and heart valve thickening. Gene transfer to a factory organ like liver may provide a lifetime source of secreted ARSB. We show that intravascular administration of adeno-associated viral vectors (AAV) 2/8-TBG-felineARSB in MPS VI cats resulted in ARSB expression up to 1 year, the last time point of the study. In newborn cats, normal circulating ARSB activity was achieved following delivery of high vector doses (6 × 10(13) genome copies (gc)/kg) whereas delivery of AAV2/8 vector doses as low as 2 × 10(12) gc/kg resulted in higher than normal serum ARSB levels in juvenile MPS VI cats. In MPS VI cats showing high serum ARSB levels, independent of the age at treatment, we observed: (i) clearance of GAG storage, (ii) improvement of long bone length, (iii) reduction of heart valve thickness, and (iv) improvement in spontaneous mobility. Thus, AAV2/ 8-mediated liver gene transfer represents a promising therapeutic strategy for MPS VI patients.
Figures

References
-
- Neufeld E., and, Muenzer J. Scriver. McGraw-Hill: New York; 2001. The mucopolysaccharidoses.
-
- Hopwood JJ., and, Morris CP. The mucopolysaccharidoses. Diagnosis, molecular genetics and treatment. Mol Biol Med. 1990;7:381–404. - PubMed
-
- Harmatz P, Giugliani R, Schwartz I, Guffon N, Teles EL, Miranda MC, et al. Enzyme replacement therapy for mucopolysaccharidosis VI: a phase 3, randomized, double-blind, placebo-controlled, multinational study of recombinant human N-acetylgalactosamine 4-sulfatase (recombinant human arylsulfatase B or rhASB) and follow-on, open-label extension study. J Pediatr. 2006;148:533–539. - PubMed
-
- Desnick RJ., and, Schuchman EH. Enzyme replacement and enhancement therapies: lessons from lysosomal disorders. Nat Rev Genet. 2002;3:954–966. - PubMed
-
- Dahms NM, Lobel P., and, Kornfeld S. Mannose 6-phosphate receptors and lysosomal enzyme targeting. J Biol Chem. 1989;264:12115–12118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

