Single-cell quantification of IL-2 response by effector and regulatory T cells reveals critical plasticity in immune response
- PMID: 21119631
- PMCID: PMC3010113
- DOI: 10.1038/msb.2010.90
Single-cell quantification of IL-2 response by effector and regulatory T cells reveals critical plasticity in immune response
Abstract
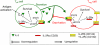
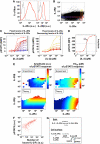
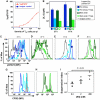
Understanding how the immune system decides between tolerance and activation by antigens requires addressing cytokine regulation as a highly dynamic process. We quantified the dynamics of interleukin-2 (IL-2) signaling in a population of T cells during an immune response by combining in silico modeling and single-cell measurements in vitro. We demonstrate that IL-2 receptor expression levels vary widely among T cells creating a large variability in the ability of the individual cells to consume, produce and participate in IL-2 signaling within the population. Our model reveals that at the population level, these heterogeneous cells are engaged in a tug-of-war for IL-2 between regulatory (T(reg)) and effector (T(eff)) T cells, whereby access to IL-2 can either increase the survival of T(eff) cells or the suppressive capacity of T(reg) cells. This tug-of-war is the mechanism enforcing, at the systems level, a core function of T(reg) cells, namely the specific suppression of survival signals for weakly activated T(eff) cells but not for strongly activated cells. Our integrated model yields quantitative, experimentally validated predictions for the manipulation of T(reg) suppression.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

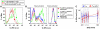


References
-
- Barthlott T, Moncrieffe H, Veldhoen M, Atkins CJ, Christensen J, O’Garra A, Stockinger B (2005) CD25+ CD4+ T cells compete with naive CD4+ T cells for IL-2 and exploit it for the induction of IL-10 production. Int Immunol 17: 279–288 - PubMed
-
- Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL (2002) CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420: 502–507 - PubMed
-
- Blinov ML, Faeder JR, Goldstein B, Hlavacek WS (2004) BioNetGen: software for rule-based modeling of signal transduction based on the interactions of molecular domains. Bioinformatics 20: 3289–3291 - PubMed
-
- Brand OJ, Lowe CE, Heward JM, Franklyn JA, Cooper JD, Todd JA, Gough SC (2007) Association of the interleukin-2 receptor alpha (IL-2Ralpha)/CD25 gene region with Graves’ disease using a multilocus test and tag SNPs. Clin Endocrinol (Oxf) 66: 508–512 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

