Substrate docking to γ-secretase allows access of γ-secretase modulators to an allosteric site
- PMID: 21119643
- PMCID: PMC3060602
- DOI: 10.1038/ncomms1129
Substrate docking to γ-secretase allows access of γ-secretase modulators to an allosteric site
Abstract
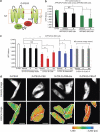
γ-Secretase generates the peptides of Alzheimer's disease, Aβ(40) and Aβ(42), by cleaving the amyloid precursor protein within its transmembrane domain. γ-Secretase also cleaves numerous other substrates, raising concerns about γ-secretase inhibitor off-target effects. Another important class of drugs, γ-secretase modulators, alter the cleavage site of γ-secretase on amyloid precursor protein, changing the Aβ(42)/Aβ(40) ratio, and are thus a promising therapeutic approach for Alzheimer's disease. However, the target for γ-secretase modulators is uncertain, with some data suggesting that they function on γ-secretase, whereas others support their binding to the amyloid precursor. In this paper we address this controversy by using a fluorescence resonance energy transfer-based assay to examine whether γ-secretase modulators alter Presenilin-1/γ-secretase conformation in intact cells in the absence of its natural substrates such as amyloid precursor protein and Notch. We report that the γ-secretase allosteric site is located within the γ-secretase complex, but substrate docking is needed for γ-secretase modulators to access this site.
Figures
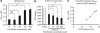

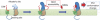
References
-
- De Strooper B. et al. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature 391, 387–390 (1998). - PubMed
-
- Wolfe M. S. et al. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature 398, 513–517 (1999). - PubMed
-
- Bitan G., Vollers S. S. & Teplow D. B. Elucidation of primary structure elements controlling early amyloid β-protein oligomerization. J. Biol. Chem. 278, 34882–34889 (2003). - PubMed
-
- Chen Y. R. & Glabe C. G. Distinct early folding and aggregation properties of Alzheimer amyloid-β peptides Aβ40 and Aβ42: stable trimer or tetramer formation by Aβ42. J. Biol. Chem. 281, 24414–24422 (2006). - PubMed
-
- Weggen S. et al. Evidence that nonsteroidal anti-inflammatory drugs decrease amyloid β42 production by direct modulation of gamma-secretase activity. J. Biol. Chem. 278, 31831–31837 (2003). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

