From genes to neural tube defects (NTDs): insights from multiscale computational modeling
- PMID: 21119766
- PMCID: PMC2929632
- DOI: 10.2976/1.3338713
From genes to neural tube defects (NTDs): insights from multiscale computational modeling
Abstract
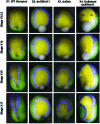
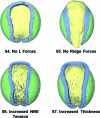
The morphogenetic movements, and the embryonic phenotypes they ultimately produce, are the consequence of a series of events that involve signaling pathways, cytoskeletal components, and cell- and tissue-level mechanical interactions. In order to better understand how these events work together in the context of amphibian neurulation, an existing multiscale computational model was augmented. Geometric data for this finite element-based mechanical model were obtained from 3D surface reconstructions of live axolotl embryos and serial sections of fixed specimens. Tissue mechanical properties were modeled using cell-based constitutive equations that include internal force generation and cell rearrangement, and equation parameters were adjusted manually to reflect biochemical changes including alterations in Shroom or the planar-cell-polarity pathway. The model indicates that neural tube defects can arise when convergent extension of the neural plate is reduced by as little as 20%, when it is eliminated on one side of the embryo, when neural ridge elevation is disrupted, when tension in the non-neural ectoderm is increased, or when the ectoderm thickness is increased. Where comparable conditions could be induced in Xenopus embryos, good agreement was found, an important step in model validation. The model reveals the neurulating embryo to be a finely tuned biomechanical system.
Figures


References
-
- Belytschko, T, Liu, W K, and Moran, B (2000). Nonlinear Finite Elements for Continua and Structures, Wiley, New York.
-
- Bordzilovskaya, N P, Dettlaff, T A, Duhon, S T, and Malacinski, G M (1989). Developmental Biology of the Axolotl, Armstrong J B and Malacinski G M (eds), Oxford University Press, New York.
LinkOut - more resources
Full Text Sources
