Pulvinar projections to the striatum and amygdala in the tree shrew
- PMID: 21120139
- PMCID: PMC2991220
- DOI: 10.3389/fnana.2010.00143
Pulvinar projections to the striatum and amygdala in the tree shrew
Abstract
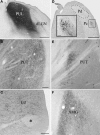
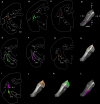
Visually guided movement is possible in the absence of conscious visual perception, a phenomenon referred to as "blindsight." Similarly, fearful images can elicit emotional responses in the absence of their conscious perception. Both capabilities are thought to be mediated by pathways from the retina through the superior colliculus (SC) and pulvinar nucleus. To define potential pathways that underlie behavioral responses to unperceived visual stimuli, we examined the projections from the pulvinar nucleus to the striatum and amygdala in the tree shrew (Tupaia belangeri), a species considered to be a prototypical primate. The tree shrew brain has a large pulvinar nucleus that contains two SC-recipient subdivisions; the dorsal (Pd) and central (Pc) pulvinar both receive topographic ("specific") projections from SC, and Pd receives an additional non-topographic ("diffuse") projection from SC (Chomsung et al., 2008). Anterograde and retrograde tract tracing revealed that both Pd and Pc project to the caudate and putamen, and Pd, but not Pc, additionally projects to the lateral amygdala. Using immunocytochemical staining for substance P (SP) and parvalbumin (PV) to reveal the patch/matrix organization of tree shrew striatum, we found that SP-rich/PV-poor patches interlock with a PV-rich/SP-poor matrix. Confocal microscopy revealed that tracer-labeled pulvino-striatal terminals preferentially innervate the matrix. Electron microscopy revealed that the postsynaptic targets of tracer-labeled pulvino-striatal and pulvino-amygdala terminals are spines, demonstrating that the pulvinar nucleus projects to the spiny output cells of the striatum matrix and the lateral amygdala, potentially relaying: (1) topographic visual information from SC to striatum to aid in guiding precise movements, and (2) non-topographic visual information from SC to the amygdala alerting the animal to potentially dangerous visual images.
Keywords: blindsight; matrix; striosome; superior colliculus; synapse.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

