Airway mucus function and dysfunction
- PMID: 21121836
- PMCID: PMC4048736
- DOI: 10.1056/NEJMra0910061
Airway mucus function and dysfunction
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures
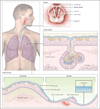



Comment in
-
Airway mucus function and dysfunction.N Engl J Med. 2011 Mar 10;364(10):978; author reply 978. doi: 10.1056/NEJMc1014719. N Engl J Med. 2011. PMID: 21388326 No abstract available.
References
-
- Thornton DJ, Sheehan JK. From mucins to mucus: toward a more coherent understanding of this essential barrier. Proc Am Thorac Soc. 2004;1:54–61. - PubMed
-
- Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol. 2008;70:459–486. - PubMed
-
- Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86:245–278. - PubMed
-
- Cone RA. Barrier properties of mucus. Adv Drug Deliv Rev. 2009;61:75–85. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
