Increased connective tissue growth factor associated with cardiac fibrosis in the mdx mouse model of dystrophic cardiomyopathy
- PMID: 21121985
- PMCID: PMC3052757
- DOI: 10.1111/j.1365-2613.2010.00750.x
Increased connective tissue growth factor associated with cardiac fibrosis in the mdx mouse model of dystrophic cardiomyopathy
Abstract
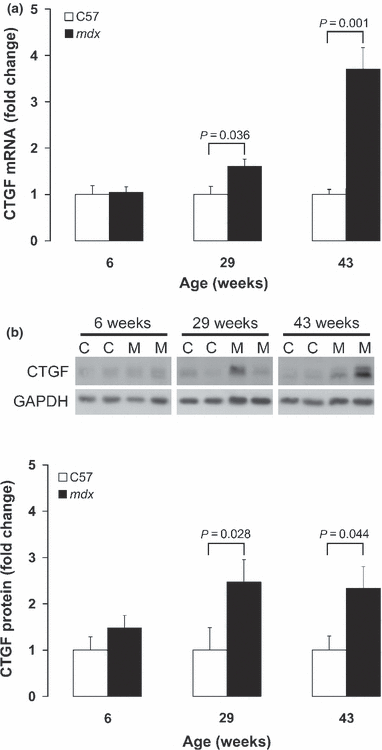
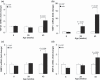
Cardiomyopathy contributes to morbidity and mortality in Duchenne muscular dystrophy (DMD), a progressive muscle-wasting disorder. A major feature of the hearts of DMD patients and the mdx mouse model of the disease is cardiac fibrosis. Connective tissue growth factor (CTGF) is involved in the fibrotic process in many organs. This study utilized the mdx mouse model to assess the role of CTGF and other extracellular matrix components during the development of fibrosis in the dystrophic heart. Left ventricular function of mdx and control mice at 6, 29 and 43 weeks was measured by echocardiography. Young (6 weeks old) mdx hearts had normal function and histology. At 29 weeks of age, mdx mice developed cardiac fibrosis and increased collagen expression. The onset of fibrosis was associated with increased CTGF transcript and protein expression. Increased intensity of CTGF immunostaining was localized to fibrotic areas in mdx hearts. The upregulation of CTGF was also concurrent with increased expression of tissue inhibitor of matrix metalloproteinases (TIMP-1). These changes persisted in 43 week old mdx hearts and were combined with impaired cardiac function and increased gene expression of transforming growth factor (TGF)-β1 and matrix metalloproteinases (MMP-2, MMP-9). In summary, an association was observed between cardiac fibrosis and increased CTGF expression in the mdx mouse heart. CTGF may be a key mediator of early and persistent fibrosis in dystrophic cardiomyopathy.
© 2010 The Authors. International Journal of Experimental Pathology © 2010 International Journal of Experimental Pathology.
Figures
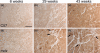
References
-
- Bauer R, Straub V, Blain A, Bushby K, MacGowan GA. Contrasting effects of steroids and angiotensin-converting-enzyme inhibitors in a mouse model of dystrophin-deficient cardiomyopathy. Eur. J. Heart Fail. 2009;11:463–471. - PubMed
-
- Bia BL, Cassidy PJ, Young ME, et al. Decreased myocardial nNOS, increased iNOS and abnormal ECGs in mouse models of Duchenne muscular dystrophy. J. Mol. Cell. Cardiol. 1999;31:1857–1862. - PubMed
-
- Bostick B, Yue Y, Long C, Duan D. Prevention of dystrophin-deficient cardiomyopathy in twenty-one-month-old carrier mice by mosaic dystrophin expression or complementary dystrophin/utrophin expression. Circ. Res. 2008;102:121–130. - PubMed
-
- Bushby K, Muntoni F, Bourke JP. 107th ENMC international workshop: the management of cardiac involvement in muscular dystrophy and myotonic dystrophy. 7th-9th June 2002, Naarden, the Netherlands. Neuromuscul. Disord. 2003;13:166–172. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

