Bacteriocyte dynamics during development of a holometabolous insect, the carpenter ant Camponotus floridanus
- PMID: 21122115
- PMCID: PMC3009655
- DOI: 10.1186/1471-2180-10-308
Bacteriocyte dynamics during development of a holometabolous insect, the carpenter ant Camponotus floridanus
Abstract
Background: The carpenter ant Camponotus floridanus harbors obligate intracellular mutualistic bacteria (Blochmannia floridanus) in specialized cells, the bacteriocytes, intercalated in their midgut tissue. The diffuse distribution of bacteriocytes over the midgut tissue is in contrast to many other insects carrying endosymbionts in specialized tissues which are often connected to the midgut but form a distinct organ, the bacteriome. C. floridanus is a holometabolous insect which undergoes a complete metamorphosis. During pupal stages a complete restructuring of the inner organs including the digestive tract takes place. So far, nothing was known about maintenance of endosymbionts during this life stage of a holometabolous insect. It was shown previously that the number of Blochmannia increases strongly during metamorphosis. This implicates an important function of Blochmannia in this developmental phase during which the animals are metabolically very active but do not have access to external food resources. Previous experiments have shown a nutritional contribution of the bacteria to host metabolism by production of essential amino acids and urease-mediated nitrogen recycling. In adult hosts the symbiosis appears to degenerate with increasing age of the animals.
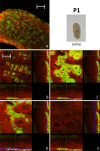
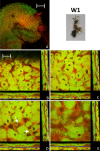
Results: We investigated the distribution and dynamics of endosymbiotic bacteria and bacteriocytes at different stages during development of the animals from larva to imago by confocal laser scanning microscopy. The number of bacteriocytes in relation to symbiont-free midgut cells varied strongly over different developmental stages. Especially during metamorphosis the relative number of bacteria-filled bacteriocytes increased strongly when the larval midgut epithelium is shed. During this developmental stage the midgut itself became a huge symbiotic organ consisting almost exclusively of cells harboring bacteria. In fact, during this phase some bacteria were also found in midgut cells other than bacteriocytes indicating a cell-invasive capacity of Blochmannia. In adult animals the number of bacteriocytes generally decreased.
Conclusions: During the life cycle of the animals the distribution of bacteriocytes and of Blochmannia endosymbionts is remarkably dynamic. Our data show how the endosymbiont is retained within the midgut tissue during metamorphosis thereby ensuring the maintenance of the intracellular endosymbiosis despite a massive reorganization of the midgut tissue. The transformation of the entire midgut into a symbiotic organ during pupal stages underscores the important role of Blochmannia for its host in particular during metamorphosis.
Figures









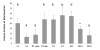

References
-
- Buchner P. Endosymbiosis of animals with plant microorganisms. Intersciences Publishers Inc. New York, N.Y; 1965.
-
- Schröder D, Deppisch H, Obermayer M, Krohne G, Stackebrandt E, Hölldobler B, Goebel W, Gross R. Intracellular endosymbiotic bacteria of Camponotus species (carpenter ants): systematics, evolution and ultrastructural characterization. Mol Microbiol. 1996;21:479–489. doi: 10.1111/j.1365-2958.1996.tb02557.x. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

