Thiol-disulfide redox dependence of heme binding and heme ligand switching in nuclear hormone receptor rev-erb{beta}
- PMID: 21123168
- PMCID: PMC3039370
- DOI: 10.1074/jbc.M110.193466
Thiol-disulfide redox dependence of heme binding and heme ligand switching in nuclear hormone receptor rev-erb{beta}
Abstract
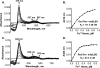
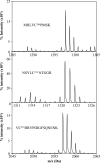
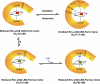
Rev-erbβ is a heme-binding nuclear hormone receptor that represses a broad spectrum of target genes involved in regulating metabolism, the circadian cycle, and proinflammatory responses. Here, we demonstrate that a thiol-disulfide redox switch controls the interaction between heme and the ligand-binding domain of Rev-erbβ. The reduced dithiol state of Rev-erbβ binds heme 5-fold more tightly than the oxidized disulfide state. By means of site-directed mutagenesis and by UV-visible and EPR spectroscopy, we also show that the ferric heme of reduced (dithiol) Rev-erbβ can undergo a redox-triggered switch from imidazole/thiol ligation (via His-568 and Cys-384, based on a prior crystal structure) to His/neutral residue ligation upon oxidation to the disulfide form. On the other hand, we find that change in the redox state of iron has no effect on heme binding to the ligand-binding domain of the protein. The low dissociation constant for the complex between Fe(3+)- or Fe(2+)-heme and the reduced dithiol state of the protein (K(d) = ∼ 20 nM) is in the range of the intracellular free heme concentration. We also determined that the Fe(2+)-heme bound to the ligand-binding domain of Rev-erbβ has high affinity for CO (K(d) = 60 nM), which replaces one of the internal ligands when bound. We suggest that this thiol-disulfide redox switch is one mechanism by which oxidative stress is linked to circadian and/or metabolic imbalance. Heme dissociation from Rev-erbβ has been shown to derepress the expression of target genes in response to changes in intracellular redox conditions. We propose that oxidative stress leads to oxidation of cysteine(s), thus releasing heme from Rev-erbβ and altering its transcriptional activity.
Figures
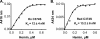

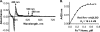
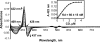
Similar articles
-
Redox Regulation of Heme Oxygenase-2 and the Transcription Factor, Rev-Erb, Through Heme Regulatory Motifs.Antioxid Redox Signal. 2018 Dec 20;29(18):1841-1857. doi: 10.1089/ars.2017.7368. Epub 2017 Nov 14. Antioxid Redox Signal. 2018. PMID: 28990415 Free PMC article. Review.
-
The heme-regulatory motif of nuclear receptor Rev-erbβ is a key mediator of heme and redox signaling in circadian rhythm maintenance and metabolism.J Biol Chem. 2017 Jul 7;292(27):11280-11299. doi: 10.1074/jbc.M117.783118. Epub 2017 May 12. J Biol Chem. 2017. PMID: 28500133 Free PMC article.
-
Ferric heme as a CO/NO sensor in the nuclear receptor Rev-Erbß by coupling gas binding to electron transfer.Proc Natl Acad Sci U S A. 2021 Jan 19;118(3):e2016717118. doi: 10.1073/pnas.2016717118. Proc Natl Acad Sci U S A. 2021. PMID: 33436410 Free PMC article.
-
The structural basis of gas-responsive transcription by the human nuclear hormone receptor REV-ERBbeta.PLoS Biol. 2009 Feb 24;7(2):e43. doi: 10.1371/journal.pbio.1000043. PLoS Biol. 2009. PMID: 19243223 Free PMC article.
-
Nuclear hormone receptors for heme: REV-ERBalpha and REV-ERBbeta are ligand-regulated components of the mammalian clock.Mol Endocrinol. 2008 Jul;22(7):1509-20. doi: 10.1210/me.2007-0519. Epub 2008 Jan 24. Mol Endocrinol. 2008. PMID: 18218725 Free PMC article. Review.
Cited by
-
Modulation of nuclear receptor function by cellular redox poise.J Inorg Biochem. 2014 Apr;133:92-103. doi: 10.1016/j.jinorgbio.2014.01.005. Epub 2014 Jan 21. J Inorg Biochem. 2014. PMID: 24495544 Free PMC article. Review.
-
Redox Regulation of Heme Oxygenase-2 and the Transcription Factor, Rev-Erb, Through Heme Regulatory Motifs.Antioxid Redox Signal. 2018 Dec 20;29(18):1841-1857. doi: 10.1089/ars.2017.7368. Epub 2017 Nov 14. Antioxid Redox Signal. 2018. PMID: 28990415 Free PMC article. Review.
-
DiGeorge critical region 8 (DGCR8) is a double-cysteine-ligated heme protein.J Biol Chem. 2011 May 13;286(19):16716-25. doi: 10.1074/jbc.M110.180844. Epub 2011 Mar 21. J Biol Chem. 2011. PMID: 21454614 Free PMC article.
-
Analytical Methods for Assessing Thiol Antioxidants in Biological Fluids: A Review.Molecules. 2024 Sep 18;29(18):4433. doi: 10.3390/molecules29184433. Molecules. 2024. PMID: 39339429 Free PMC article. Review.
-
Misalignment of Circadian Rhythms in Diet-Induced Obesity.Adv Exp Med Biol. 2024;1460:27-71. doi: 10.1007/978-3-031-63657-8_2. Adv Exp Med Biol. 2024. PMID: 39287848 Review.
References
-
- Bain D. L., Heneghan A. F., Connaghan-Jones K. D., Miura M. T. (2007) Annu. Rev. Physiol. 69, 201–220 - PubMed
-
- Olefsky J. M. (2001) J. Biol. Chem. 276, 36863–36864 - PubMed
-
- Retnakaran R., Flock G., Giguère V. (1994) Mol. Endocrinol. 8, 1234–1244 - PubMed
-
- Duez H., Staels B. (2008) FEBS Lett. 582, 19–25 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

