Fc gammaRIIIb triggers raft-dependent calcium influx in IgG-mediated responses in human neutrophils
- PMID: 21123174
- PMCID: PMC3030356
- DOI: 10.1074/jbc.M110.169516
Fc gammaRIIIb triggers raft-dependent calcium influx in IgG-mediated responses in human neutrophils
Abstract
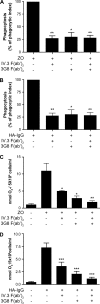
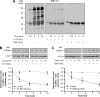
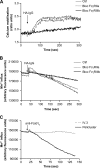
Human neutrophils constitutively express a unique combination of FcγRs, namely FcγRIIa and FcγRIIIb. Numerous lines of evidence support the concept that these FcγRs generate only partially characterized intracellular signals. However, despite the fact that both receptors are likely to be engaged simultaneously in a physiological setting, no recent publications have investigated the distinct, although partially convergent, results of their joint activation in IgG-dependent responses. To examine the significance of the co-expression of FcγRIIa and FcγRIIIb on human neutrophils, we analyzed the neutrophil responses to stimuli that engage these FcγRs, namely the phagocytosis of human IgG-opsonized zymosan and the responses to heat-aggregated IgGs. Blocking antibodies to either FcγR significantly decreased the phagocytic index and the stimulated production of superoxide anions. Both receptors are required for optimal IgG-dependent responses by human neutrophils. On the other hand, only blocking antibodies to FcγRIIIb, but not to FcγRIIa, inhibited the mobilization of calcium in response to heat-aggregated IgGs. Furthermore, phagocytosis of IgG-opsonized zymosan by human neutrophils required an extracellular influx of calcium that was blocked only by antibodies against FcγRIIIb. We also observed that this calcium influx as well as the IgG-dependent phagocytosis were dependent on the integrity of the plasma membrane detergent-resistant microdomains to which both isoforms were recruited following stimulation by heat-aggregated IgGs. These data clarify the mechanisms that regulate the FcγRs constitutively expressed on human neutrophils, describe a specific contribution of FcγRIIIb at the level of the mobilization of calcium, and provide evidence for a crucial role of detergent-resistant microdomains in this process.
Figures
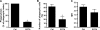

Similar articles
-
The Antibody Receptor Fc Gamma Receptor IIIb Induces Calcium Entry via Transient Receptor Potential Melastatin 2 in Human Neutrophils.Front Immunol. 2021 May 13;12:657393. doi: 10.3389/fimmu.2021.657393. eCollection 2021. Front Immunol. 2021. PMID: 34054821 Free PMC article.
-
Haplotypes of FcγRIIa and FcγRIIIb polymorphic variants influence IgG-mediated responses in neutrophils.J Immunol. 2014 Mar 15;192(6):2715-21. doi: 10.4049/jimmunol.1203570. Epub 2014 Feb 19. J Immunol. 2014. PMID: 24554771
-
Fc gamma receptors activate different protein kinase C isoforms in human neutrophils.J Leukoc Biol. 2025 Apr 23;117(4):qiaf019. doi: 10.1093/jleuko/qiaf019. J Leukoc Biol. 2025. PMID: 39946245
-
The immunoglobulin, IgG Fc receptor and complement triangle in autoimmune diseases.Immunobiology. 2012 Nov;217(11):1067-79. doi: 10.1016/j.imbio.2012.07.015. Immunobiology. 2012. PMID: 22964232 Review.
-
Fc gamma receptor mediated activation of human polymorphonuclear neutrophils (PMN).Arch Immunol Ther Exp (Warsz). 1992;40(1):65-6. Arch Immunol Ther Exp (Warsz). 1992. PMID: 1485829 Review. No abstract available.
Cited by
-
Differential Use of Human Neutrophil Fcγ Receptors for Inducing Neutrophil Extracellular Trap Formation.J Immunol Res. 2016;2016:2908034. doi: 10.1155/2016/2908034. Epub 2016 Jan 14. J Immunol Res. 2016. PMID: 27034964 Free PMC article.
-
CIN85 modulates the down-regulation of Fc gammaRIIa expression and function by c-Cbl in a PKC-dependent manner in human neutrophils.J Biol Chem. 2011 Apr 29;286(17):15073-84. doi: 10.1074/jbc.M110.213660. Epub 2011 Mar 3. J Biol Chem. 2011. PMID: 21372129 Free PMC article.
-
Fc gamma receptors in respiratory syncytial virus infections: implications for innate immunity.Rev Med Virol. 2014 Jan;24(1):55-70. doi: 10.1002/rmv.1773. Epub 2013 Nov 14. Rev Med Virol. 2014. PMID: 24227634 Free PMC article. Review.
-
Force-dependent calcium signaling and its pathway of human neutrophils on P-selectin in flow.Protein Cell. 2017 Feb;8(2):103-113. doi: 10.1007/s13238-016-0364-4. Epub 2017 Jan 18. Protein Cell. 2017. PMID: 28097631 Free PMC article.
-
The Antibody Receptor Fc Gamma Receptor IIIb Induces Calcium Entry via Transient Receptor Potential Melastatin 2 in Human Neutrophils.Front Immunol. 2021 May 13;12:657393. doi: 10.3389/fimmu.2021.657393. eCollection 2021. Front Immunol. 2021. PMID: 34054821 Free PMC article.
References
-
- Schmidt R. E., Gessner J. E. (2005) Immunol. Lett. 100, 56–67 - PubMed
-
- Willcocks L. C., Smith K. G., Clatworthy M. R. (2009) Expert. Rev. Mol. Med. 11, e24. - PubMed
-
- Selvaraj P., Fifadara N., Nagarajan S., Cimino A., Wang G. (2004) Immunol. Res. 29, 219–230 - PubMed
-
- Hogarth P. M. (2002) Curr. Opin. Immunol. 14, 798–802 - PubMed
-
- Hulett M. D., Hogarth P. M. (1994) Adv. Immunol. 57, 1–127 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

