LIMP-2 links late phagosomal trafficking with the onset of the innate immune response to Listeria monocytogenes: a role in macrophage activation
- PMID: 21123180
- PMCID: PMC3030339
- DOI: 10.1074/jbc.M110.146761
LIMP-2 links late phagosomal trafficking with the onset of the innate immune response to Listeria monocytogenes: a role in macrophage activation
Abstract
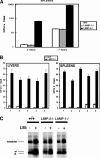
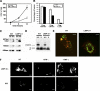
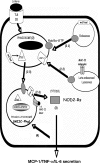
The innate immune response to Listeria monocytogenes depends on phagosomal bacterial degradation by macrophages. Here, we describe the role of LIMP-2, a lysosomal type III transmembrane glycoprotein and scavenger-like protein, in Listeria phagocytosis. LIMP-2-deficient mice display a macrophage-related defect in Listeria innate immunity. They produce less acute phase pro-inflammatory cytokines/chemokines, MCP-1, TNF-α, and IL-6 but normal levels of IL-12, IL-10, and IFN-γ and a 25-fold increase in susceptibility to Listeria infection. This macrophage defect results in a low listericidal potential, poor response to TNF-α activation signals, impaired phago-lysosome transformation into antigen-processing compartments, and uncontrolled LM cytosolic growth that fails to induce normal levels of acute phase pro-inflammatory cytokines. LIMP-2 transfection of CHO cells confirmed that LIMP-2 participates in the degradation of Listeria within phagosomes, controls the late endosomal/lysosomal fusion machinery, and is linked to the activation of Rab5a. Therefore, the role of LIMP-2 appears to be connected to the TNF-α-dependent and early activation of Listeria macrophages through internal signals linking the regulation of late trafficking events with the onset of the innate Listeria immune response.
Figures

Similar articles
-
Dissociation of innate immune responses in microglia infected with Listeria monocytogenes.Glia. 2014 Feb;62(2):233-46. doi: 10.1002/glia.22602. Epub 2013 Dec 6. Glia. 2014. PMID: 24311463 Free PMC article.
-
Bacterial ligands generated in a phagosome are targets of the cytosolic innate immune system.PLoS Pathog. 2007 Mar;3(3):e51. doi: 10.1371/journal.ppat.0030051. PLoS Pathog. 2007. PMID: 17397264 Free PMC article.
-
Impaired macrophage listericidal and cytokine activities are responsible for the rapid death of Listeria monocytogenes-infected IFN-gamma receptor-deficient mice.J Immunol. 1997 Jun 1;158(11):5297-304. J Immunol. 1997. PMID: 9164949
-
Innate immune pathways triggered by Listeria monocytogenes and their role in the induction of cell-mediated immunity.Adv Immunol. 2012;113:135-56. doi: 10.1016/B978-0-12-394590-7.00002-6. Adv Immunol. 2012. PMID: 22244582 Review.
-
Roles of factor increasing monocytopoiesis (FIM) and macrophage activation in host resistance to Listeria monocytogenes.Infection. 1988;16 Suppl 2:S137-40. doi: 10.1007/BF01639736. Infection. 1988. PMID: 3138186 Review.
Cited by
-
SCARB2/LIMP-2 Regulates IFN Production of Plasmacytoid Dendritic Cells by Mediating Endosomal Translocation of TLR9 and Nuclear Translocation of IRF7.J Immunol. 2015 May 15;194(10):4737-49. doi: 10.4049/jimmunol.1402312. Epub 2015 Apr 10. J Immunol. 2015. PMID: 25862818 Free PMC article.
-
Bioanalysis of eukaryotic organelles.Chem Rev. 2013 Apr 10;113(4):2733-811. doi: 10.1021/cr300354g. Chem Rev. 2013. PMID: 23570618 Free PMC article. Review. No abstract available.
-
Pleiotropic Roles of Scavenger Receptors in Circadian Retinal Phagocytosis: A New Function for Lysosomal SR-B2/LIMP-2 at the RPE Cell Surface.Int J Mol Sci. 2022 Mar 22;23(7):3445. doi: 10.3390/ijms23073445. Int J Mol Sci. 2022. PMID: 35408805 Free PMC article.
-
Dissociation of innate immune responses in microglia infected with Listeria monocytogenes.Glia. 2014 Feb;62(2):233-46. doi: 10.1002/glia.22602. Epub 2013 Dec 6. Glia. 2014. PMID: 24311463 Free PMC article.
-
Abnormal Processing of Autophagosomes in Transformed B Lymphocytes from SCARB2-Deficient Subjects.Biores Open Access. 2013 Feb;2(1):40-6. doi: 10.1089/biores.2012.0265. Biores Open Access. 2013. PMID: 23515316 Free PMC article.
References
-
- Kobayashi K. S., Chamaillard M., Ogura Y., Henegariu O., Inohara N., Nuñez G., Flavell R. A. (2005) Science 307, 731–734 - PubMed
-
- Crombie R., Silverstein R. (1998) J. Biol. Chem. 273, 4855–4863 - PubMed
-
- Agaisse H., Burrack L. S., Philips J. A., Rubin E. J., Perrimon N., Higgins D. E. (2005) Science 309, 1248–1251 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

