A novel sequence-based antigenic distance measure for H1N1, with application to vaccine effectiveness and the selection of vaccine strains
- PMID: 21123189
- PMCID: PMC3038458
- DOI: 10.1093/protein/gzq105
A novel sequence-based antigenic distance measure for H1N1, with application to vaccine effectiveness and the selection of vaccine strains
Abstract
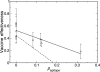
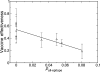
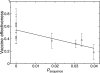
H1N1 influenza causes substantial seasonal illness and was the subtype of the 2009 influenza pandemic. Precise measures of antigenic distance between the vaccine and circulating virus strains help researchers design influenza vaccines with high vaccine effectiveness. We here introduce a sequence-based method to predict vaccine effectiveness in humans. Historical epidemiological data show that this sequence-based method is as predictive of vaccine effectiveness as hemagglutination inhibition assay data from ferret animal model studies. Interestingly, the expected vaccine effectiveness is greater against H1N1 than H3N2, suggesting a stronger immune response against H1N1 than H3N2. The evolution rate of hemagglutinin in H1N1 is also shown to be greater than that in H3N2, presumably due to greater immune selection pressure.
Figures


Similar articles
-
Antigenic stability of H1N1 pandemic vaccines correlates with vaccine strain.Vaccine. 2011 Feb 11;29(8):1529-33. doi: 10.1016/j.vaccine.2010.12.120. Epub 2011 Jan 4. Vaccine. 2011. PMID: 21211583
-
Implementing sequence-based antigenic distance calculation into immunological shape space model.BMC Bioinformatics. 2020 Jun 19;21(1):256. doi: 10.1186/s12859-020-03594-3. BMC Bioinformatics. 2020. PMID: 32560624 Free PMC article.
-
Antigenic cartography of H1N1 influenza viruses using sequence-based antigenic distance calculation.BMC Bioinformatics. 2018 Feb 12;19(1):51. doi: 10.1186/s12859-018-2042-4. BMC Bioinformatics. 2018. PMID: 29433425 Free PMC article.
-
H3N2 influenza viruses in humans: Viral mechanisms, evolution, and evaluation.Hum Vaccin Immunother. 2018;14(8):1840-1847. doi: 10.1080/21645515.2018.1462639. Epub 2018 May 14. Hum Vaccin Immunother. 2018. PMID: 29641358 Free PMC article. Review.
-
Advances in protein subunit vaccines against H1N1/09 influenza.Front Immunol. 2024 Nov 22;15:1499754. doi: 10.3389/fimmu.2024.1499754. eCollection 2024. Front Immunol. 2024. PMID: 39650643 Free PMC article. Review.
Cited by
-
Antigenic drift and epidemiological severity of seasonal influenza in Canada.Sci Rep. 2022 Sep 17;12(1):15625. doi: 10.1038/s41598-022-19996-7. Sci Rep. 2022. PMID: 36115880 Free PMC article.
-
Effects of Prior Influenza Exposure on Immunogenicity of Influenza Vaccine.Open Forum Infect Dis. 2020 May 26;7(8):ofaa181. doi: 10.1093/ofid/ofaa181. eCollection 2020 Aug. Open Forum Infect Dis. 2020. PMID: 32818136 Free PMC article.
-
Antigenicity prediction and vaccine recommendation of human influenza virus A (H3N2) using convolutional neural networks.Hum Vaccin Immunother. 2020 Nov 1;16(11):2690-2708. doi: 10.1080/21645515.2020.1734397. Epub 2020 Aug 4. Hum Vaccin Immunother. 2020. PMID: 32750260 Free PMC article.
-
Influenza evolution and H3N2 vaccine effectiveness, with application to the 2014/2015 season.Protein Eng Des Sel. 2016 Aug;29(8):309-15. doi: 10.1093/protein/gzw017. Epub 2016 Jun 16. Protein Eng Des Sel. 2016. PMID: 27313229 Free PMC article.
-
Oligomerization of bacterially expressed H1N1 recombinant hemagglutinin contributes to protection against viral challenge.Sci Rep. 2018 Aug 7;8(1):11856. doi: 10.1038/s41598-018-30079-4. Sci Rep. 2018. PMID: 30087372 Free PMC article.
References
-
- Belongia E., Kieke B., Coleman L., et al. J. Am. Med. Assoc. 2008;299:2381–2384.
-
- Brown I.H., Harris P.A., McCauley J.W., Alexander D.J. J. Gen. Virol. 1998;79:2947–2955. - PubMed
-
- Caton A.J., Brownlee G.G., Yewdell J.W., Gerhard W. Cell. 1982;31:417–427. - PubMed
-
- Centers for Disease Control and Prevention (CDC) MMWR Morb. Mortal. Wkly. Rep. 2009a;58:1241–1245. - PubMed
-
- Centers for Disease Control and Prevention (CDC) MMWR Morb. Mortal. Wkly. Rep. 2009b;58:1009–1012. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

