Effect of 7,8-dihydroxyflavone, a small-molecule TrkB agonist, on emotional learning
- PMID: 21123312
- PMCID: PMC3770732
- DOI: 10.1176/appi.ajp.2010.10030326
Effect of 7,8-dihydroxyflavone, a small-molecule TrkB agonist, on emotional learning
Abstract
Objective: Despite increasing awareness of the many important roles played by brain-derived neurotrophic factor (BDNF) activation of TrkB, a fuller understanding of this system and the use of potential TrkB-acting therapeutic agents has been limited by the lack of any identified small-molecule TrkB agonists that fully mimic the actions of BDNF at brain TrkB receptors in vivo. However, 7,8-dihydroxyflavone (7,8-DHF) has recently been identified as a specific TrkB agonist that crosses the blood-brain barrier after oral or intraperitoneal administration. The authors combined pharmacological, biochemical, and behavioral approaches in a preclinical study examining the role of 7,8-DHF in modulating emotional memory in mice.
Method: The authors first examined the ability of systemic 7,8-DHF to activate TrkB receptors in the amygdala. They then examined the effects of systemic 7,8-DHF on acquisition and extinction of conditioned fear, using specific and well-characterized BDNF-dependent learning paradigms in several models using naive mice and mice with prior traumatic stress exposure.
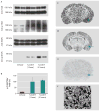
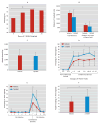
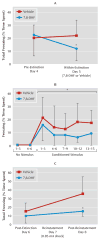
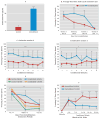
Results: Amygdala TrkB receptors, which have previously been shown to be required for emotional learning, were activated by systemic 7,8-DHF (at 5 mg/kg i.p.). 7,8-DHF enhanced both the acquisition of fear and its extinction. It also appeared to rescue an extinction deficit in mice with a history of immobilization stress.
Conclusions: These data suggest that 7,8-DHF may be an excellent agent for use in understanding the effects of TrkB activation in learning and memory paradigms and may be attractive for use in reversing learning and extinction deficits associated with psychopathology.
Conflict of interest statement
The authors report no financial relationships with commercial interests.
Figures
Comment in
-
Toward neurotrophin-based therapeutics.Am J Psychiatry. 2011 Feb;168(2):114-6. doi: 10.1176/appi.ajp.2010.10111677. Am J Psychiatry. 2011. PMID: 21297044 No abstract available.
References
-
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. - PubMed
-
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–535. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

