Crucial role for prion protein membrane anchoring in the neuroinvasion and neural spread of prion infection
- PMID: 21123371
- PMCID: PMC3028874
- DOI: 10.1128/JVI.02167-10
Crucial role for prion protein membrane anchoring in the neuroinvasion and neural spread of prion infection
Abstract
In nature prion diseases are usually transmitted by extracerebral prion infection, but clinical disease results only after invasion of the central nervous system (CNS). Prion protein (PrP), a host-encoded glycosylphosphatidylinositol (GPI)-anchored membrane glycoprotein, is necessary for prion infection and disease. Here, we investigated the role of the anchoring of PrP on prion neuroinvasion by studying various inoculation routes in mice expressing either anchored or anchorless PrP. In control mice with anchored PrP, intracerebral or sciatic nerve inoculation resulted in rapid CNS neuroinvasion and clinical disease (154 to 156 days), and after tongue, ocular, intravenous, or intraperitoneal inoculation, CNS neuroinvasion was only slightly slower (193 to 231 days). In contrast, in anchorless PrP mice, these routes resulted in slow and infrequent CNS neuroinvasion. Only intracerebral inoculation caused brain PrPres, a protease-resistant isoform of PrP, and disease in both types of mice. Thus, anchored PrP was an essential component for the rapid neural spread and CNS neuroinvasion of prion infection.
Figures
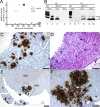


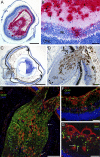

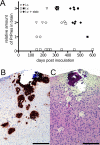
References
-
- Atherton, S. S., C. K. Newell, M. Y. Kanter, and S. W. Cousins. 1991. Retinitis in euthymic mice following inoculation of murine cytomegalovirus (MCMV) via the supraciliary route. Curr. Eye Res. 10:667-677. - PubMed
-
- Baldauf, E., M. Beekes, and H. Diringer. 1997. Evidence for an alternative direct route of access for the scrapie agent to the brain bypassing the spinal cord. J. Gen. Virol. 78:1187-1197. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

