BCR/ABL stimulates WRN to promote survival and genomic instability
- PMID: 21123451
- PMCID: PMC3032814
- DOI: 10.1158/0008-5472.CAN-10-1066
BCR/ABL stimulates WRN to promote survival and genomic instability
Abstract
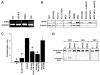
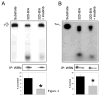
BCR/ABL-transformed chronic myeloid leukemia (CML) cells accumulate numerous DNA double-strand breaks (DSB) induced by reactive oxygen species (ROS) and genotoxic agents. To repair these lesions BCR/ABL stimulate unfaithful DSB repair pathways, homologous recombination repair (HRR), nonhomologous end-joining (NHEJ), and single-strand annealing (SSA). Here, we show that BCR/ABL enhances the expression and increase nuclear localization of WRN (mutated in Werner syndrome), which is required for processing DSB ends during the repair. Other fusion tyrosine kinases (FTK), such as TEL/ABL, TEL/JAK2, TEL/PDGFβR, and NPM/ALK also elevate WRN. BCR/ABL induces WRN mRNA and protein expression in part by c-MYC-mediated activation of transcription and Bcl-xL-dependent inhibition of caspase-dependent cleavage, respectively. WRN is in complex with BCR/ABL resulting in WRN tyrosine phosphorylation and stimulation of its helicase and exonuclease activities. Activated WRN protects BCR/ABL-positive cells from the lethal effect of oxidative and genotoxic stresses, which causes DSBs. In addition, WRN promotes unfaithful recombination-dependent repair mechanisms HRR and SSA, and enhances the loss of DNA bases during NHEJ in leukemia cells. In summary, we postulate that BCR/ABL-mediated stimulation of WRN modulates the efficiency and fidelity of major DSB repair mechanisms to protect leukemia cells from apoptosis and to facilitate genomic instability.
Figures

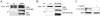



Similar articles
-
Up-regulation of WRN and DNA ligase IIIalpha in chronic myeloid leukemia: consequences for the repair of DNA double-strand breaks.Blood. 2008 Aug 15;112(4):1413-23. doi: 10.1182/blood-2007-07-104257. Epub 2008 Jun 4. Blood. 2008. PMID: 18524993 Free PMC article.
-
BCR/ABL and other kinases from chronic myeloproliferative disorders stimulate single-strand annealing, an unfaithful DNA double-strand break repair.Cancer Res. 2008 Sep 1;68(17):6884-8. doi: 10.1158/0008-5472.CAN-08-1101. Cancer Res. 2008. PMID: 18757400 Free PMC article.
-
BCR-ABL stimulates mutagenic homologous DNA double-strand break repair via the DNA-end-processing factor CtIP.Carcinogenesis. 2011 Jan;32(1):27-34. doi: 10.1093/carcin/bgq216. Epub 2010 Oct 25. Carcinogenesis. 2011. PMID: 20974687
-
BCR-ABL: a multi-faceted promoter of DNA mutation in chronic myelogeneous leukemia.Leukemia. 2010 Jun;24(6):1105-12. doi: 10.1038/leu.2010.67. Epub 2010 May 6. Leukemia. 2010. PMID: 20445577 Free PMC article. Review.
-
Genomic instability: The cause and effect of BCR/ABL tyrosine kinase.Curr Hematol Malig Rep. 2007 May;2(2):69-74. doi: 10.1007/s11899-007-0010-6. Curr Hematol Malig Rep. 2007. PMID: 20425353 Review.
Cited by
-
RECQL1 and WRN DNA repair helicases: potential therapeutic targets and proliferative markers against cancers.Front Genet. 2015 Jan 9;5:441. doi: 10.3389/fgene.2014.00441. eCollection 2014. Front Genet. 2015. PMID: 25620975 Free PMC article. Review.
-
Potential mechanisms of disease progression and management of advanced-phase chronic myeloid leukemia.Leuk Lymphoma. 2014 Jul;55(7):1451-62. doi: 10.3109/10428194.2013.845883. Epub 2013 Nov 12. Leuk Lymphoma. 2014. PMID: 24050507 Free PMC article. Review.
-
PARP1 inhibitor eliminated imatinib-refractory chronic myeloid leukemia cells in bone marrow microenvironment conditions.Leuk Lymphoma. 2019 Jan;60(1):262-264. doi: 10.1080/10428194.2018.1471602. Epub 2018 Jun 22. Leuk Lymphoma. 2019. PMID: 29932782 Free PMC article. No abstract available.
-
Concomitant inhibition of the thioredoxin system and nonhomologous DNA repair potently sensitizes Philadelphia-positive lymphoid leukemia to tyrosine kinase inhibitors.Hemasphere. 2024 Mar 14;8(3):e56. doi: 10.1002/hem3.56. eCollection 2024 Mar. Hemasphere. 2024. PMID: 38486859 Free PMC article.
-
Differential prognostic impact of stratified additional chromosome abnormalities on disease progression among Malaysian chronic myeloid leukemia patients undergoing treatment with imatinib mesylate.Front Oncol. 2022 Aug 8;12:720845. doi: 10.3389/fonc.2022.720845. eCollection 2022. Front Oncol. 2022. PMID: 36003793 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

