Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury
- PMID: 21123492
- PMCID: PMC3064142
- DOI: 10.1152/ajprenal.00546.2010
Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury
Abstract
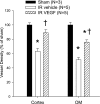
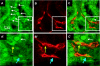
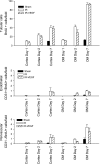
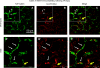
Acute kidney injury induces the loss of renal microvessels, but the fate of endothelial cells and the mechanism of potential vascular endothelial growth factor (VEGF)-mediated protection is unknown. Cumulative cell proliferation was analyzed in the kidney of Sprague-Dawley rats following ischemia-reperfusion (I/R) injury by repetitive administration of BrdU (twice daily) and colocalization in endothelial cells with CD31 or cablin. Proliferating endothelial cells were undetectable for up to 2 days following I/R and accounted for only ∼1% of BrdU-positive cells after 7 days. VEGF-121 preserved vascular loss following I/R but did not affect proliferation of endothelial, perivascular cells or tubular cells. Endothelial mesenchymal transition states were identified by localizing endothelial markers (CD31, cablin, or infused tomato lectin) with the fibroblast marker S100A4. Such structures were prominent within 6 h and sustained for at least 7 days following I/R. A Tie-2-cre transgenic crossed with a yellow fluorescent protein (YFP) reporter mouse was used to trace the fate of endothelial cells and demonstrated interstititial expansion of YFP-positive cells colocalizing with S100A4 and smooth muscle actin following I/R. The interstitial expansion of YFP cells was attenuated by VEGF-121. Multiphoton imaging of transgenic mice revealed the alteration of YFP-positive vascular cells associated with blood vessels characterized by limited perfusion in vivo. Taken together, these data indicate that vascular dropout post-AKI results from endothelial phenotypic transition combined with an impaired regenerative capacity, which may contribute to progressive chronic kidney disease.
Figures






References
-
- Askenazi D, Feig D, Graham N, Hui-Stickle S, Goldstein SL. 3–5 Year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int 69: 184–189, 2006 - PubMed
-
- Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int 72: 151–156, 2007 - PubMed
-
- Basile DP, Donohoe DL, Roethe K, Mattson DL. Chronic renal hypoxia following ischemia/reperfusion injury: effects of l-arginine on hypoxia and secondary damage. Am J Physiol Renal Physiol 284: F338–F348, 2003 - PubMed
-
- Basile DP, Donohoe DL, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281: F887–F899, 2001 - PubMed
-
- Basile DP, Fredrich K, Chelladurai B, Leonard EC, Parrish AR. Renal ischemia reperfusion inhibits VEGF expression and induces ADAMTS-1, a novel VEGF inhibitor. Am J Physiol Renal Physiol 294: F928–F936, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

