Purified hexameric Epstein-Barr virus-encoded BARF1 protein for measuring anti-BARF1 antibody responses in nasopharyngeal carcinoma patients
- PMID: 21123521
- PMCID: PMC3067345
- DOI: 10.1128/CVI.00193-10
Purified hexameric Epstein-Barr virus-encoded BARF1 protein for measuring anti-BARF1 antibody responses in nasopharyngeal carcinoma patients
Abstract
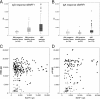
WHO type III nasopharyngeal carcinoma (NPC) is highly prevalent in Indonesia and 100% associated with Epstein-Barr virus (EBV). NPC tumor cells express viral proteins, including BARF1, which is secreted and is considered to have oncogenic and immune-modulating properties. Recently, we found conserved mutations in the BARF1 gene in NPC isolates. This study describes the expression and purification of NPC-derived BARF1 and analyzes humoral immune responses against prototype BARF1 (B95-8) and purified native hexameric BARF1 in sera of Indonesian NPC patients (n = 155) compared to healthy EBV-positive (n = 56) and EBV-negative (n = 16) individuals. BARF1 (B95-8) expressed in Escherichia coli and baculovirus, as well as BARF1-derived peptides, did not react with IgG or IgA antibodies in NPC. Purified native hexameric BARF1 protein isolated from culture medium was used in enzyme-linked immunosorbent assay (ELISA) and revealed relatively weak IgG and IgA responses in human sera, although it had strong antibody responses to other EBV proteins. Higher IgG reactivity was found in NPC patients (P = 0.015) than in regional Indonesian controls or EBV-negative individuals (P < 0.001). IgA responses to native BARF1 were marginal. NPC sera with the highest IgG responses to hexameric BARF1 in ELISA showed detectable reactivity with denatured BARF1 by immunoblotting. In conclusion, BARF1 has low immunogenicity for humoral responses and requires native conformation for antibody binding. The presence of antibodies against native BARF1 in the blood of NPC patients provides evidence that the protein is expressed and secreted as a hexameric protein in NPC patients.
Figures



Similar articles
-
Single-assay combination of Epstein-Barr Virus (EBV) EBNA1- and viral capsid antigen-p18-derived synthetic peptides for measuring anti-EBV immunoglobulin G (IgG) and IgA antibody levels in sera from nasopharyngeal carcinoma patients: options for field screening.J Clin Microbiol. 2006 Apr;44(4):1459-67. doi: 10.1128/JCM.44.4.1459-1467.2006. J Clin Microbiol. 2006. PMID: 16597877 Free PMC article.
-
Evaluation of commercial EBV RecombLine assay for diagnosis of nasopharyngeal carcinoma.J Clin Virol. 2008 Aug;42(4):343-52. doi: 10.1016/j.jcv.2008.03.006. Epub 2008 May 1. J Clin Virol. 2008. PMID: 18455473
-
Diagnostic value of measuring Epstein-Barr virus (EBV) DNA load and carcinoma-specific viral mRNA in relation to anti-EBV immunoglobulin A (IgA) and IgG antibody levels in blood of nasopharyngeal carcinoma patients from Indonesia.J Clin Microbiol. 2005 Jul;43(7):3066-73. doi: 10.1128/JCM.43.7.3066-3073.2005. J Clin Microbiol. 2005. PMID: 16002393 Free PMC article.
-
Diagnostic Value of Serum Epstein-Barr Virus Capsid Antigen-IgA for Nasopharyngeal Carcinoma: a Meta-Analysis Based on 21 Studies.Clin Lab. 2016;62(6):1155-66. doi: 10.7754/clin.lab.2015.151122. Clin Lab. 2016. PMID: 27468579 Review.
-
Understanding the interplay between host immunity and Epstein-Barr virus in NPC patients.Emerg Microbes Infect. 2015 Mar;4(3):e20. doi: 10.1038/emi.2015.20. Epub 2015 Mar 25. Emerg Microbes Infect. 2015. PMID: 26038769 Free PMC article. Review.
Cited by
-
Epstein-Barr virus-encoded BARF1 promotes proliferation of gastric carcinoma cells through regulation of NF-κB.J Virol. 2013 Oct;87(19):10515-23. doi: 10.1128/JVI.00955-13. Epub 2013 Jul 3. J Virol. 2013. PMID: 23824821 Free PMC article.
-
A Distinct Anti-EBV DNase Profile in Patients with Undifferentiated Nasopharyngeal Carcinoma Compared to Classical Antigens.Viruses. 2023 Oct 26;15(11):2158. doi: 10.3390/v15112158. Viruses. 2023. PMID: 38005835 Free PMC article.
-
BamHI-A rightward frame 1, an Epstein-Barr virus-encoded oncogene and immune modulator.Rev Med Virol. 2013 Nov;23(6):367-83. doi: 10.1002/rmv.1758. Epub 2013 Aug 31. Rev Med Virol. 2013. PMID: 23996634 Free PMC article. Review.
-
Therapeutic implications of Epstein-Barr virus infection for the treatment of nasopharyngeal carcinoma.Ther Clin Risk Manag. 2014 Sep 5;10:721-36. doi: 10.2147/TCRM.S47434. eCollection 2014. Ther Clin Risk Manag. 2014. PMID: 25228810 Free PMC article. Review.
-
BARF1 gene silencing triggers caspase-dependent mitochondrial apoptosis in Epstein-Barr virus-positive malignant cells.J Biosci. 2015 Mar;40(1):41-51. doi: 10.1007/s12038-015-9502-z. J Biosci. 2015. PMID: 25740140
References
-
- Chang, E. T., and H. O. Adami. 2006. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol. Biomarkers Prev. 15:1765-1777. - PubMed
-
- Chang, M. S., et al. 2005. Cell-cycle regulators, bcl-2 and NF-kappaB in Epstein-Barr virus-positive gastric carcinomas. Int. J. Oncol. 27:1265-1272. - PubMed
-
- Decaussin, G., F. Sbih-Lammali, M. de Turenne-Tessier, A. Bouguermouh, and T. Ooka. 2000. Expression of BARF1 gene encoded by Epstein-Barr virus in nasopharyngeal carcinoma biopsies. Cancer Res. 60:5584-5588. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

