Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex
- PMID: 21123555
- PMCID: PMC3004756
- DOI: 10.1523/JNEUROSCI.1731-10.2010
Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex
Abstract
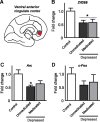
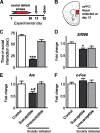
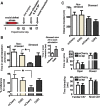
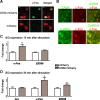
Brain stimulation and imaging studies in humans have highlighted a key role for the prefrontal cortex in clinical depression; however, it remains unknown whether excitation or inhibition of prefrontal cortical neuronal activity is associated with antidepressant responses. Here, we examined cellular indicators of functional activity, including the immediate early genes (IEGs) zif268 (egr1), c-fos, and arc, in the prefrontal cortex of clinically depressed humans obtained postmortem. We also examined these genes in the ventral portion of the medial prefrontal cortex (mPFC) of mice after chronic social defeat stress, a mouse model of depression. In addition, we used viral vectors to overexpress channel rhodopsin 2 (a light-activated cation channel) in mouse mPFC to optogenetically drive "burst" patterns of cortical firing in vivo and examine the behavioral consequences. Prefrontal cortical tissue derived from clinically depressed humans displayed significant reductions in IEG expression, consistent with a deficit in neuronal activity within this brain region. Mice subjected to chronic social defeat stress exhibited similar reductions in levels of IEG expression in mPFC. Interestingly, some of these changes were not observed in defeated mice that escape the deleterious consequences of the stress, i.e., resilient animals. In those mice that expressed a strong depressive-like phenotype, i.e., susceptible animals, optogenetic stimulation of mPFC exerted potent antidepressant-like effects, without affecting general locomotor activity, anxiety-like behaviors, or social memory. These results indicate that the activity of the mPFC is a key determinant of depression-like behavior, as well as antidepressant responses.
Figures
References
-
- Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458:1025–1029. - PubMed
-
- Baeg EH, Kim YB, Jang J, Kim HT, Mook-Jung I, Jung MW. Fast spiking and regular spiking neural correlates of fear conditioning in the medial prefrontal cortex of the rat. Cereb Cortex. 2001;11:441–451. - PubMed
-
- Barbas H. Anatomic basis of cognitive-emotional interactions in the primate prefrontal cortex. Neurosci Biobehav Rev. 1995;19:499–510. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
