Ventral tegmental afferents in stress-induced reinstatement: the role of cAMP response element-binding protein
- PMID: 21123561
- PMCID: PMC3075606
- DOI: 10.1523/JNEUROSCI.2827-10.2010
Ventral tegmental afferents in stress-induced reinstatement: the role of cAMP response element-binding protein
Abstract
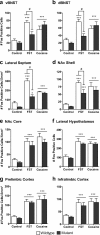
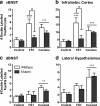
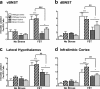
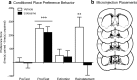
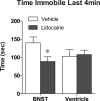
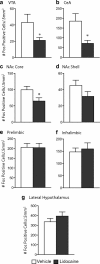
The transcription factor cAMP response element-binding protein (CREB) is required for stress- but not drug-induced reinstatement of cocaine conditioned place preference. To reveal the neural circuitry associated with this CREB dependence, we injected a retrograde tracer into the ventral tegmental area (VTA) and identified afferents that were activated after stress or cocaine exposure in both naive and cocaine-conditioned mice. Neuronal activation, as assessed by Fos expression, was greatly reduced in the dorsal and ventral bed nucleus of the stria terminalis (BNST), lateral septum, and nucleus accumbens shell in mice lacking CREB (CREBαΔ mice) after a 6 min swim stress but not after cocaine exposure (20 mg/kg). Additionally, activation of VTA afferent neurons in the ventral BNST and the infralimbic cortex in CREBαΔ mice was blunted in response to stress. This pattern of neuronal activation persisted in mice that were conditioned to a cocaine place preference procedure before stress exposure. Furthermore, lidocaine inactivation (0.4 μl, 4%) studies demonstrated the necessity of BNST activation for swim-stress-induced reinstatement of cocaine-conditioned reward. Together, the present studies demonstrate that CREB is required for the activation of a unique circuit that converges on the dopamine reward pathway to elicit reinstatement of drug reward and points to the BNST as a key intersection between stress and reward circuits.
Figures






References
-
- Blundell J, Adamec R. The NMDA receptor antagonist CPP blocks the effects of predator stress on pCREB in brain regions involved in fearful and anxious behavior. Brain Res. 2007;1136:59–76. - PubMed
-
- Brown SA, Vik PW, McQuaid JR, Patterson TL, Irwin MR, Grant I. Severity of psychosocial stress and outcome of alcoholism treatment. J Abnorm Psychol. 1990;99:344–348. - PubMed
-
- Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA. Stress, vulnerability and adult alcohol relapse. J Stud Alcohol. 1995;56:538–545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
