Leptin rapidly improves glucose homeostasis in obese mice by increasing hypothalamic insulin sensitivity
- PMID: 21123564
- PMCID: PMC3697436
- DOI: 10.1523/JNEUROSCI.3202-10.2010
Leptin rapidly improves glucose homeostasis in obese mice by increasing hypothalamic insulin sensitivity
Abstract
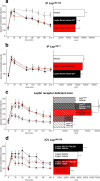
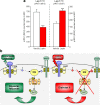
Obesity is associated with resistance to the actions of both leptin and insulin via mechanisms that remain incompletely understood. To investigate whether leptin resistance per se contributes to insulin resistance and impaired glucose homeostasis, we investigated the effect of acute leptin administration on glucose homeostasis in normal as well as leptin- or leptin receptor-deficient mice. In hyperglycemic, leptin-deficient Lep(ob/ob) mice, leptin acutely and potently improved glucose metabolism, before any change of body fat mass, via a mechanism involving the p110α and β isoforms of phosphatidylinositol-3-kinase (PI3K). Unlike insulin, however, the anti-diabetic effect of leptin occurred independently of phospho-AKT, a major downstream target of PI3K, and instead involved enhanced sensitivity of the hypothalamus to insulin action upstream of PI3K, through modulation of IRS1 (insulin receptor substrate 1) phosphorylation. These data suggest that leptin resistance, as occurs in obesity, reduces the hypothalamic response to insulin and thereby impairs peripheral glucose homeostasis, contributing to the development of type 2 diabetes.
Figures


Similar articles
-
Inactivation of class II PI3K-C2α induces leptin resistance, age-dependent insulin resistance and obesity in male mice.Diabetologia. 2016 Jul;59(7):1503-1512. doi: 10.1007/s00125-016-3963-y. Epub 2016 Apr 30. Diabetologia. 2016. PMID: 27138914 Free PMC article.
-
Ablation of ghrelin receptor in leptin-deficient ob/ob mice has paradoxical effects on glucose homeostasis when compared with ablation of ghrelin in ob/ob mice.Am J Physiol Endocrinol Metab. 2012 Aug 1;303(3):E422-31. doi: 10.1152/ajpendo.00576.2011. Epub 2012 Jun 5. Am J Physiol Endocrinol Metab. 2012. PMID: 22669248 Free PMC article.
-
Long-term effects of central leptin and resistin on body weight, insulin resistance, and beta-cell function and mass by the modulation of hypothalamic leptin and insulin signaling.Endocrinology. 2008 Feb;149(2):445-54. doi: 10.1210/en.2007-0754. Epub 2007 Nov 8. Endocrinology. 2008. PMID: 17991727
-
Hypothalamic Insulin Resistance in Obesity: Effects on Glucose Homeostasis.Neuroendocrinology. 2017;104(4):364-381. doi: 10.1159/000455865. Epub 2017 Jan 26. Neuroendocrinology. 2017. PMID: 28122381 Review.
-
Akt isoforms and glucose homeostasis - the leptin connection.Trends Endocrinol Metab. 2011 Feb;22(2):66-73. doi: 10.1016/j.tem.2010.09.003. Epub 2010 Oct 12. Trends Endocrinol Metab. 2011. PMID: 20947368 Free PMC article. Review.
Cited by
-
Central GLP-2 enhances hepatic insulin sensitivity via activating PI3K signaling in POMC neurons.Cell Metab. 2013 Jul 2;18(1):86-98. doi: 10.1016/j.cmet.2013.06.014. Cell Metab. 2013. PMID: 23823479 Free PMC article.
-
Exercise training and de-training effects on serum leptin and TNF-α in high fat induced diabetic rats.Diabetol Metab Syndr. 2021 May 26;13(1):57. doi: 10.1186/s13098-021-00676-x. Diabetol Metab Syndr. 2021. PMID: 34039404 Free PMC article.
-
Harnessing the Power of Leptin: The Biochemical Link Connecting Obesity, Diabetes, and Cognitive Decline.Front Aging Neurosci. 2022 Apr 22;14:861350. doi: 10.3389/fnagi.2022.861350. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35527735 Free PMC article. Review.
-
Pyruvate induces torpor in obese mice.Proc Natl Acad Sci U S A. 2018 Jan 23;115(4):810-815. doi: 10.1073/pnas.1717507115. Epub 2018 Jan 8. Proc Natl Acad Sci U S A. 2018. PMID: 29311303 Free PMC article.
-
The LIM domain only 4 protein is a metabolic responsive inhibitor of protein tyrosine phosphatase 1B that controls hypothalamic leptin signaling.J Neurosci. 2013 Jul 31;33(31):12647-55. doi: 10.1523/JNEUROSCI.0746-13.2013. J Neurosci. 2013. PMID: 23904601 Free PMC article.
References
-
- Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. - PubMed
-
- Choudhury AI, Heffron H, Smith MA, Al-Qassab H, Xu AW, Selman C, Simmgen M, Clements M, Claret M, Maccoll G, Bedford DC, Hisadome K, Diakonov I, Moosajee V, Bell JD, Speakman JR, Batterham RL, Barsh GS, Ashford ML, Withers DJ. The role of insulin receptor substrate 2 in hypothalamic and beta cell function. J Clin Invest. 2005;115:940–950. - PMC - PubMed
-
- Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O'Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–884. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
