Caloric restriction experience reprograms stress and orexigenic pathways and promotes binge eating
- PMID: 21123586
- PMCID: PMC3034235
- DOI: 10.1523/JNEUROSCI.1955-10.2010
Caloric restriction experience reprograms stress and orexigenic pathways and promotes binge eating
Abstract
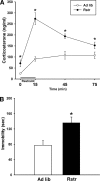
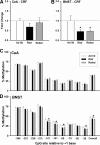
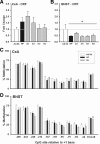
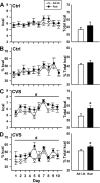
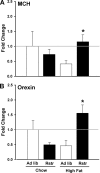
Long-term weight management by dieting has a high failure rate. Pharmacological targets have focused on appetite reduction, although less is understood as to the potential contributions of the stress state during dieting in long-term behavioral modification. In a mouse model of moderate caloric restriction in which a 10-15% weight loss similar to human dieting is produced, we examined physiological and behavioral stress measures. After 3 weeks of restriction, mice showed significant increases in immobile time in a tail suspension test and stress-induced corticosterone levels. Increased stress was associated with brain region-specific alterations of corticotropin-releasing factor expression and promoter methylation, changes that were not normalized with refeeding. Similar outcomes were produced by high-fat diet withdrawal, an additional component of human dieting. In examination of long-term behavioral consequences, previously restricted mice showed a significant increase in binge eating of a palatable high-fat food during stress exposure. Orexigenic hormones, melanin-concentrating hormone (MCH) and orexin, were significantly elevated in response to the high-fat diet only in previously restricted mice. Furthermore, administration of the MCH receptor-1 antagonist GSK-856464 [4-(4-ethyl-5-methylsulfanyl-1,2,4-triazol-3-yl)pyridine] significantly reduced total caloric intake in these mice during high-fat access. These results reveal reprogramming of key central pathways involved in regulating stress responsivity and orexigenic drives by moderate caloric restriction experience. In humans, such changes would be expected to reduce treatment success by promoting behaviors resulting in weight regain, and suggest that management of stress during dieting may be beneficial in long-term maintenance.
Figures
Comment in
-
Systems neuroscience: the stress of dieting.Nat Rev Neurosci. 2011 Feb;12(2):65. doi: 10.1038/nrn2985. Nat Rev Neurosci. 2011. PMID: 21309096 No abstract available.
Similar articles
-
Orexin signaling mediates the antidepressant-like effect of calorie restriction.J Neurosci. 2008 Mar 19;28(12):3071-5. doi: 10.1523/JNEUROSCI.5584-07.2008. J Neurosci. 2008. PMID: 18354010 Free PMC article.
-
Effects of CB1 and CRF1 receptor antagonists on binge-like eating in rats with limited access to a sweet fat diet: lack of withdrawal-like responses.Physiol Behav. 2012 Sep 10;107(2):231-42. doi: 10.1016/j.physbeh.2012.06.017. Epub 2012 Jul 6. Physiol Behav. 2012. PMID: 22776620 Free PMC article.
-
A new animal model of binge eating: key synergistic role of past caloric restriction and stress.Physiol Behav. 2002 Sep;77(1):45-54. doi: 10.1016/s0031-9384(02)00809-0. Physiol Behav. 2002. PMID: 12213501
-
Leptin signaling, adiposity, and energy balance.Ann N Y Acad Sci. 2002 Jun;967:379-88. doi: 10.1111/j.1749-6632.2002.tb04293.x. Ann N Y Acad Sci. 2002. PMID: 12079865 Review.
-
Dopamine and binge eating behaviors.Pharmacol Biochem Behav. 2010 Nov;97(1):25-33. doi: 10.1016/j.pbb.2010.04.016. Epub 2010 Apr 24. Pharmacol Biochem Behav. 2010. PMID: 20417658 Free PMC article. Review.
Cited by
-
Late-life time-restricted feeding and exercise differentially alter healthspan in obesity.Aging Cell. 2019 Aug;18(4):e12966. doi: 10.1111/acel.12966. Epub 2019 May 21. Aging Cell. 2019. PMID: 31111669 Free PMC article.
-
Orexin 1 receptor antagonists in compulsive behavior and anxiety: possible therapeutic use.Front Neurosci. 2014 Feb 13;8:26. doi: 10.3389/fnins.2014.00026. eCollection 2014. Front Neurosci. 2014. PMID: 24592206 Free PMC article. Review.
-
Neuroendocrine circuits governing energy balance and stress regulation: functional overlap and therapeutic implications.Cell Metab. 2014 Jun 3;19(6):910-25. doi: 10.1016/j.cmet.2014.01.020. Epub 2014 Mar 13. Cell Metab. 2014. PMID: 24630812 Free PMC article. Review.
-
Dairy food consumption and meal-induced cortisol response interacted to influence weight loss in overweight women undergoing a 12-week, meal-controlled, weight loss intervention.J Nutr. 2013 Jan;143(1):46-52. doi: 10.3945/jn.112.166355. Epub 2012 Nov 28. J Nutr. 2013. PMID: 23190756 Free PMC article. Clinical Trial.
-
Obesity-insulin targeted genes in the 3p26-25 region in human studies and LG/J and SM/J mice.Metabolism. 2012 Aug;61(8):1129-41. doi: 10.1016/j.metabol.2012.01.008. Epub 2012 Mar 3. Metabolism. 2012. PMID: 22386932 Free PMC article.
References
-
- Amigo I, Fernández C. Effects of diets and their role in weight control. Psychol Health Med. 2007;12:321–327. - PubMed
-
- Beck B, Kozak R, Moar KM, Mercer JG. Hypothalamic orexigenic peptides are overexpressed in young Long-Evans rats after early life exposure to fat-rich diets. Biochem Biophys Res Commun. 2006;342:452–458. - PubMed
-
- Bishop NA, Guarente L. Genetic links between diet and lifespan: shared mechanisms from yeast to humans. Nat Rev Genet. 2007;8:835–844. - PubMed
-
- Brownell KD, Rodin J. Medical, metabolic, and psychological effects of weight cycling. Arch Intern Med. 1994;154:1325–1330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
