Degeneration and regeneration of the intervertebral disc: lessons from development
- PMID: 21123625
- PMCID: PMC3008962
- DOI: 10.1242/dmm.006403
Degeneration and regeneration of the intervertebral disc: lessons from development
Abstract
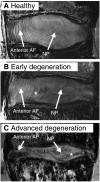
Degeneration of the intervertebral discs, a process characterized by a cascade of cellular, biochemical, structural and functional changes, is strongly implicated as a cause of low back pain. Current treatment strategies for disc degeneration typically address the symptoms of low back pain without treating the underlying cause or restoring mechanical function. A more in-depth understanding of disc degeneration, as well as opportunities for therapeutic intervention, can be obtained by considering aspects of intervertebral disc development. Development of the intervertebral disc involves the coalescence of several different cell types through highly orchestrated and complex molecular interactions. The resulting structures must function synergistically in an environment that is subjected to continuous mechanical perturbation throughout the life of an individual. Early postnatal changes, including altered cellularity, vascular regression and altered extracellular matrix composition, might set the disc on a slow course towards symptomatic degeneration. In this Perspective, we review the pathogenesis and treatment of intervertebral disc degeneration in the context of disc development. Within this scope, we examine how model systems have advanced our understanding of embryonic morphogenesis and associated molecular signaling pathways, in addition to the postnatal changes to the cellular, nutritional and mechanical microenvironment. We also discuss the current status of biological therapeutic strategies that promote disc regeneration and repair, and how lessons from development might provide clues for their refinement.
Figures
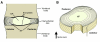

References
-
- Acaroglu E.R., Iatridis J.C., Setton L.A., Foster R.J., Mow V.C., Weidenbaum M. (1995). Degeneration and aging affect the tensile behavior of human lumbar anulus fibrosus. Spine 20, 2690–2701 - PubMed
-
- Adams D.S., Keller R., Koehl M.A. (1990). The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development 110, 115–130 - PubMed
-
- Adams M.A., Roughley P.J. (2006). What is intervertebral disc degeneration, and what causes it? Spine 31, 2151–2161 - PubMed
-
- Adams M.A., McNally D.S., Dolan P. (1996). ‘Stress’ distributions inside intervertebral discs. The effects of age and degeneration. J. Bone Joint Surg. Br. 78, 965–972 - PubMed
-
- Aguiar D.J., Johnson S.L., Oegema T.R. (1999). Notochordal cells interact with nucleus pulposus cells: regulation of proteoglycan synthesis. Exp. Cell Res. 246, 129–137 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

