CB1 modulation of temporally distinct synaptic facilitation among local circuit interneurons mediated by N-type calcium channels in CA1
- PMID: 21123660
- PMCID: PMC3074412
- DOI: 10.1152/jn.00831.2010
CB1 modulation of temporally distinct synaptic facilitation among local circuit interneurons mediated by N-type calcium channels in CA1
Abstract
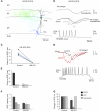
One of the critical factors in determining network behavior of neurons is the influence of local circuit connections among interneurons. The short-term synaptic plasticity and the subtype of presynaptic calcium channels used at local circuit connections of inhibitory interneurons in CA1 were investigated using dual whole-cell recordings combined with biocytin and double immunofluorescence labeling in acute slices of P18- to 21-day-old rat stratum radiatum (SR) and stratum lacunosum molecular (SLM). Two forms of temporally distinct synaptic facilitation were observed among interneuron connections involving presynaptic cholecystokinin (CCK)-positive cells in SR, frequency-dependent facilitation, and a delayed onset of release (45-80 ms) with subsequent facilitation (DORF). Inhibition at both these synapses was under tonic cannabinoid-type 1 (CB1) receptor activity. DORF synapses did not display conventional release-dependent properties; however, blocking CB1 receptors with antagonist AM-251 (10 μM) altered the synaptic transmission to frequency-dependent depression with a fast onset of release (2-4 ms). Presynaptic CCK-negative interneurons in SLM elicited inhibitory postsynaptic potentials (IPSPs) insensitive to CB1 receptor pharmacology displayed frequency-dependent depression. Release of GABA at facilitating synapses was solely mediated via N-type presynaptic calcium channels, whereas depressing synapses utilized P/Q-type channels. These data reveal two distinct models of neurotransmitter release patterns among interneuron circuits that correlate with the subtype of presynaptic calcium channel. These data suggest that endocannabinoids act via CB1 receptors to selectively modulate N-type calcium channels to alter signal transmission.
Figures




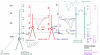
References
-
- Ali AB. Presynaptic inhibition of GABAA receptor-mediated unitary IPSPs by cannabinoid receptors at synapses between CCK-positive interneurons in rat hippocampus. J Neurophysiol 98: 861–869, 2007 - PubMed
-
- Ali AB, Bannister AP, Thomson AM. IPSPs elicited in CA1 pyramidal cells by putative basket cells in slices of adult rat hippocampus. Eur J Neurosci 11: 1741–1753, 1999 - PubMed
-
- Ali AB, Nelson C. Distinct Ca2+ channels mediate transmitter release at excitatory synapses displaying different dynamic properties in rat neocortex. Cereb Cortex 6: 386–393, 2006 - PubMed
-
- Ali AB, Todorova M. Asynchronous release of GABA via tonic cannabinoid receptor activation at identified interneuron synapses in rat CA1. Eur J Neurosci 31: 3–12, 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

