Increased matriptase zymogen activation in inflammatory skin disorders
- PMID: 21123732
- PMCID: PMC3063967
- DOI: 10.1152/ajpcell.00403.2010
Increased matriptase zymogen activation in inflammatory skin disorders
Abstract
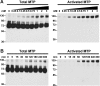
Matriptase, a type 2 transmembrane serine protease, and its inhibitor hepatocyte growth factor activator inhibitor (HAI)-1 are required for normal epidermal barrier function, and matriptase activity is tightly regulated during this process. We therefore hypothesized that this protease system might be deregulated in skin disease. To test this, we examined the level and activation state of matriptase in examples of 23 human skin disorders. We first examined matriptase and HAI-1 protein distribution in normal epidermis. Matriptase was detected at high levels at cell-cell junctions in the basal layer and spinous layers but was present at minimal levels in the granular layer. HAI-1 was distributed in a similar pattern, except that high-level expression was retained in the granular layer. This pattern of expression was retained in most skin disorders. We next examined the distribution of activated matriptase. Although activated matriptase is not detected in normal epidermis, a dramatic increase is seen in keratinocytes at the site of inflammation in 16 different skin diseases. To gain further evidence that activation is associated with inflammatory stimuli, we challenged HaCaT cells with acidic pH or H(2)O(2) and observed matriptase activation. These findings suggest that inflammation-associated reactive oxygen species and tissue acidity may enhance matriptase activation in some skin diseases.
Figures







References
-
- Alef T, Torres S, Hausser I, Metze D, Tursen U, Lestringant GG, Hennies HC. Ichthyosis, follicular atrophoderma, and hypotrichosis caused by mutations in ST14 is associated with impaired profilaggrin processing. J Invest Dermatol 129: 862–869, 2009 - PubMed
-
- Avrahami L, Maas S, Pasmanik-Chor M, Rainshtein L, Magal N, Smitt J, van Marle J, Shohat M, Basel-Vanagaite L. Autosomal recessive ichthyosis with hypotrichosis syndrome: further delineation of the phenotype. Clin Genet 74: 47–53, 2008 - PubMed
-
- Basel-Vanagaite L, Attia R, Ishida-Yamamoto A, Rainshtein L, Ben AD, Lurie R, Pasmanik-Chor M, Indelman M, Zvulunov A, Saban S, Magal N, Sprecher E, Shohat M. Autosomal recessive ichthyosis with hypotrichosis caused by a mutation in ST14, encoding type II transmembrane serine protease matriptase. Am J Hum Genet 80: 467–477, 2007 - PMC - PubMed
-
- Benaud C, Dickson RB, Lin CY. Regulation of the activity of matriptase on epithelial cell surfaces by a blood-derived factor. Eur J Biochem 268: 1439–1447, 2001 - PubMed
-
- Benaud C, Oberst M, Hobson JP, Spiegel S, Dickson RB, Lin CY. Sphingosine 1-phosphate, present in serum-derived lipoproteins, activates matriptase. J Biol Chem 277: 10539–10546, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

