Hypoxia. 2. Hypoxia regulates cellular metabolism
- PMID: 21123733
- PMCID: PMC3063979
- DOI: 10.1152/ajpcell.00485.2010
Hypoxia. 2. Hypoxia regulates cellular metabolism
Abstract
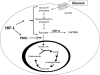
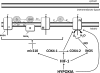
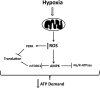
Adaptation to lowering oxygen levels (hypoxia) requires coordinated downregulation of metabolic demand and supply to prevent a mismatch in ATP utilization and production that might culminate in a bioenergetic collapse. Hypoxia diminishes ATP utilization by downregulating protein translation and the activity of the Na-K-ATPase. Hypoxia diminishes ATP production in part by lowering the activity of the electron transport chain through activation of the transcription factor hypoxia-inducible factor-1. The decrease in electron transport limits the overproduction of reactove oxygen species during hypoxia and slows the rate of oxygen depletion to prevent anoxia. In this review, we discuss these mechanisms that diminish metabolic supply and demand for adaptation to hypoxia.
Figures


References
-
- Aragones J, Schneider M, Van Geyte K, Fraisl P, Dresselaers T, Mazzone M, Dirkx R, Zacchigna S, Lemieux H, Jeoung NH, Lambrechts D, Bishop T, Lafuste P, Diez-Juan A, Harten SK, Van Noten P, De Bock K, Willam C, Tjwa M, Grosfeld A, Navet R, Moons L, Vandendriessche T, Deroose C, Wijeyekoon B, Nuyts J, Jordan B, Silasi-Mansat R, Lupu F, Dewerchin M, Pugh C, Salmon P, Mortelmans L, Gallez B, Gorus F, Buyse J, Sluse F, Harris RA, Gnaiger E, Hespel P, Van Hecke P, Schuit F, Van Veldhoven P, Ratcliffe P, Baes M, Maxwell P, Carmeliet P. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet 40: 170–180, 2008 - PubMed
-
- Arai AE, Grauer SE, Anselone CG, Pantely GA, Bristow JD. Metabolic adaptation to a gradual reduction in myocardial blood flow. Circulation 92: 244–252, 1995 - PubMed
-
- Arai AE, Pantely GA, Anselone CG, Bristow J, Bristow JD. Active downregulation of myocardial energy requirements during prolonged moderate ischemia in swine. Circ Res 69: 1458–1469, 1991 - PubMed
-
- Arsham AM, Howell JJ, Simon MC. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J Biol Chem 278: 29655–29660, 2003 - PubMed
-
- Aw TY, Andersson BS, Jones DP. Suppression of mitochondrial respiratory function after short-term anoxia. Am J Physiol Cell Physiol 252: C362–C368, 1987 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

