Replacing the enzyme alpha-L-iduronidase at birth ameliorates symptoms in the brain and periphery of dogs with mucopolysaccharidosis type I
- PMID: 21123810
- PMCID: PMC3075726
- DOI: 10.1126/scitranslmed.3001380
Replacing the enzyme alpha-L-iduronidase at birth ameliorates symptoms in the brain and periphery of dogs with mucopolysaccharidosis type I
Abstract
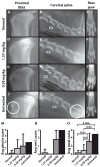
Mucopolysaccharidosis type I (MPS I) is a lysosomal storage disease caused by loss of activity of α-l-iduronidase and attendant accumulation of the glycosaminoglycans dermatan sulfate and heparan sulfate. Current treatments are suboptimal and do not address residual disease including corneal clouding, skeletal deformities, valvular heart disease, and cognitive impairment. We treated neonatal dogs with MPS I with intravenous recombinant α-l-iduronidase replacement therapy at the conventional 0.58 mg/kg or a higher 1.57 mg/kg weekly dose for 56 to 81 weeks. In contrast to previous results in animals and patients treated at a later age, the dogs failed to mount an antibody response to enzyme therapy, consistent with the induction of immune tolerance in neonates. The higher dose of enzyme led to complete normalization of lysosomal storage in the liver, spleen, lung, kidney, synovium, and myocardium, as well as in the hard-to-treat mitral valve. Cardiac biochemistry and function were restored, and there were improvements in skeletal disease as shown by clinical and radiographic assessments. Glycosaminoglycan levels in the brain were normalized after intravenous enzyme therapy, in the presence or absence of intrathecal administration of recombinant α-l-iduronidase. Histopathological evidence of glycosaminoglycan storage in the brain was ameliorated with the higher-dose intravenous therapy and was further improved by combining intravenous and intrathecal therapy. These findings argue that neonatal testing and early treatment of patients with MPS I may more effectively treat this disease.
Conflict of interest statement
Figures





References
-
- Bunge S, Clements PR, Byers S, Kleijer WJ, Brooks DA, Hopwood JJ. Genotype-phenotype correlations in mucopolysaccharidosis type I using enzyme kinetics, immunoquantification and in vitro turnover studies. Biochim Biophys Acta. 1998;1407:249–256. - PubMed
-
- Neufeld EF, Muenzer J. In: The Metabolic and Molecular Bases of Inherited Disease. Scriver CR, Beaudet AL, Sly WS, Valle D, editors. McGraw-Hill, Health Professions Division; New York: 2001. pp. 3421–3452.
-
- Soper BW, Duffy TM, Lessard MD, Jude CD, Schuldt AJ, Vogler CA, Levy B, Barker JE. Transplanted ER-MP12hi20-58med/hi myeloid progenitors produce resident macrophages from marrow that are therapeutic for lysosomal storage disease. Blood Cells Mol Dis. 2004;32:199–213. - PubMed
-
- Whitley CB, Belani KG, Chang PN, Summers CG, Blazar BR, Tsai MY, Latchaw RE, Ramsay NK, Kersey JH. Long-term outcome of Hurler syndrome following bone marrow transplantation. Am J Med Genet. 1993;46:209–218. - PubMed
-
- Pastores GM, Arn P, Beck M, Clarke JT, Guffon N, Kaplan P, Muenzer J, Norato DY, Shapiro E, Thomas J, Viskochil D, Wraith JE. The MPS I registry: design, methodology, and early findings of a global disease registry for monitoring patients with Mucopolysaccharidosis Type I. Mol Genet Metab. 2007;91:37–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

