Guidance for removal of fetal bovine serum from cryopreserved heart valve processing
- PMID: 21123998
- PMCID: PMC3202934
- DOI: 10.1159/000321166
Guidance for removal of fetal bovine serum from cryopreserved heart valve processing
Abstract
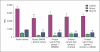
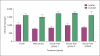
Bovine serum is commonly used in cryopreservation of allogeneic heart valves; however, bovine serum carries a risk of product adulteration by contamination with bovine-derived infectious agents. In this study, we compared fresh and cryopreserved porcine valves that were processed by 1 of 4 cryopreservation formulations, 3 of which were serum-free and 1 that utilized bovine serum with 1.4 M dimethylsulfoxide. In the first serum-free group, bovine serum was simply removed from the cryopreservation formulation. The second serum-free formulation had a higher cryoprotectant concentration, i.e. 2 M dimethylsulfoxide, in combination with a serum-free solution. A colloid, dextran 40, was added to the third serum-free group with 2 M dimethylsulfoxide due to theoretical concerns that removal of serum might increase the incidence of tissue cracking. Upon rewarming, the valves were inspected and subjected to a battery of tests. Gross pathology revealed conduit cracking in 1 of 98 frozen heart valves. Viability data for the cryopreserved groups versus the fresh group demonstrated a loss of viability in half of the comparisons (p < 0.05). No significant differences were observed between any of the cryopreserved groups, with or without bovine serum. Neither routine histology, autofluorescence-based multiphoton imaging nor semiquantitative second-harmonic generation microscopy of extracellular matrix components revealed any statistically significant differences. Biomechanics analyses also revealed no significant differences. Our results demonstrate that bovine serum can be safely removed from heart valve processing and that a colloid to prevent cracking was not required. This study provides guidance for the assessment of changes in cryopreservation procedures for tissues.
Copyright © 2010 S. Karger AG, Basel.
Figures


Similar articles
-
Development of a simplified ice-free cryopreservation method for heart valves employing VS83, an 83% cryoprotectant formulation.Biopreserv Biobank. 2012 Dec;10(6):479-84. doi: 10.1089/bio.2012.0006. Biopreserv Biobank. 2012. PMID: 24845133
-
Ice-free cryopreservation of heart valve allografts: better extracellular matrix preservation in vivo and preclinical results.Cell Tissue Bank. 2012 Dec;13(4):663-71. doi: 10.1007/s10561-011-9288-7. Epub 2012 Jan 3. Cell Tissue Bank. 2012. PMID: 22212702
-
Impact of cryopreservation on extracellular matrix structures of heart valve leaflets.Ann Thorac Surg. 2006 Mar;81(3):918-26. doi: 10.1016/j.athoracsur.2005.09.016. Ann Thorac Surg. 2006. PMID: 16488695
-
[Cellular viability and immune response in homologous cryopreserved cardiac valves].Minerva Cardioangiol. 1997 May;45(5):235-44. Minerva Cardioangiol. 1997. PMID: 9273475 Review. Italian.
-
Cellular biology of cryopreserved allograft valves.J Med Invest. 2001 Aug;48(3-4):123-32. J Med Invest. 2001. PMID: 11694951 Review.
Cited by
-
Cryopreservation of dermal fibroblasts and keratinocytes in hydroxyethyl starch-based cryoprotectants.BMC Biotechnol. 2016 Dec 1;16(1):85. doi: 10.1186/s12896-016-0315-4. BMC Biotechnol. 2016. PMID: 27903244 Free PMC article.
-
Removal of transmissible spongiform encephalopathy prion from large volumes of cell culture media supplemented with fetal bovine serum by using hollow fiber anion-exchange membrane chromatography.PLoS One. 2015 Apr 13;10(4):e0122300. doi: 10.1371/journal.pone.0122300. eCollection 2015. PLoS One. 2015. PMID: 25874629 Free PMC article.
-
Impact of storage solution formulation during refrigerated storage upon chondrocyte viability and cartilage matrix.Cells Tissues Organs. 2014;199(1):51-8. doi: 10.1159/000363134. Epub 2014 Aug 21. Cells Tissues Organs. 2014. PMID: 25171188 Free PMC article.
-
Impact of Hypothermia upon Chondrocyte Viability and Cartilage Matrix Permeability after 1 Month of Refrigerated Storage.Transfus Med Hemother. 2011 Dec;38(6):387-392. doi: 10.1159/000334595. Epub 2011 Nov 14. Transfus Med Hemother. 2011. PMID: 22403523 Free PMC article.
-
Evaluation of sericin as a fetal bovine serum-replacing cryoprotectant during freezing of human mesenchymal stromal cells and human osteoblast-like cells.Biopreserv Biobank. 2014 Apr;12(2):99-105. doi: 10.1089/bio.2013.0078. Biopreserv Biobank. 2014. PMID: 24749876 Free PMC article.
References
-
- Asher D.M. The transmissible spongiform encephalopathy agents, concerns and responses of United States regulatory agencies in maintaining the safety of biologics. Dev Biol Stand. 1999;100:103–118. - PubMed
-
- Bradley R. Bovine spongiform encephalopathy and its relationship to the variant form of Creutzfeldt-Jakob disease. Contrib Microbiol. 2004;11:146–185. - PubMed
-
- Brockbank K.G.M. Current practices in heart valve preservation. Part 1. Bioprocessing. 2007;6:29–36.
-
- Brockbank K.G.M., Lightfoot F.G., Song Y.C., Taylor M.J. Interstitial ice formation in cryopreserved homografts: a possible cause of tissue deterioration and calcification in vivo. J Heart Valve Dis. 2000;9:200–206. - PubMed

