Are circulating metabolites important in drug-drug interactions?: Quantitative analysis of risk prediction and inhibitory potency
- PMID: 21124313
- PMCID: PMC3474849
- DOI: 10.1038/clpt.2010.252
Are circulating metabolites important in drug-drug interactions?: Quantitative analysis of risk prediction and inhibitory potency
Erratum in
- Clin Pharmacol Ther. 2011 Mar;89(3):468
Abstract
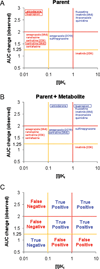
The potential of metabolites to contribute to drug-drug interactions (DDIs) is not well defined. The aim of this study was to determine the quantitative role of circulating metabolites in inhibitory DDIs in vivo. The area under the plasma concentration-time curve (AUC) data related to at least one circulating metabolite was available for 71% of the 102 inhibitor drugs identified. Of the 80 metabolites characterized at steady state, 78% had AUCs >10% of that of the parent drug. A comparison of the inhibitor concentration/inhibition constant ([I]/K(i)) ratios of metabolites and the respective parent drugs showed that 17 of the 21 (80%) reversible inhibitors studied had metabolites that were likely to contribute to in vivo DDIs, with some metabolites predicted to have inhibitory effects greater than those of the parent drug. The in vivo drug interaction risks associated with amiodarone, bupropion, and sertraline could be identified from in vitro data only, when data pertaining to metabolites were included in the predictions. In conclusion, cytochrome P450 (CYP) inhibitors often have circulating metabolites that contribute to clinically observed CYP inhibition.
Conflict of interest statement
None
Figures
Similar articles
-
Drug metabolites as cytochrome p450 inhibitors: a retrospective analysis and proposed algorithm for evaluation of the pharmacokinetic interaction potential of metabolites in drug discovery and development.Drug Metab Dispos. 2013 Dec;41(12):2047-55. doi: 10.1124/dmd.113.052241. Epub 2013 Jun 21. Drug Metab Dispos. 2013. PMID: 23792812
-
Do Inhibitory Metabolites Impact DDI Risk Assessment? Analysis of in vitro and in vivo Data from NDA Reviews Between 2013 and 2018.Clin Pharmacol Ther. 2021 Aug;110(2):452-463. doi: 10.1002/cpt.2259. Epub 2021 May 8. Clin Pharmacol Ther. 2021. PMID: 33835478 Free PMC article.
-
A perspective on the contribution of metabolites to drug-drug interaction potential: the need to consider both circulating levels and inhibition potency.Drug Metab Dispos. 2013 Mar;41(3):536-40. doi: 10.1124/dmd.112.048892. Epub 2012 Nov 9. Drug Metab Dispos. 2013. PMID: 23143892
-
Qualitative analysis of the role of metabolites in inhibitory drug-drug interactions: literature evaluation based on the metabolism and transport drug interaction database.Chem Res Toxicol. 2009 Feb;22(2):294-8. doi: 10.1021/tx800491e. Chem Res Toxicol. 2009. PMID: 19216580 Free PMC article. Review.
-
The role of metabolites in predicting drug-drug interactions: focus on irreversible cytochrome P450 inhibition.Curr Opin Drug Discov Devel. 2010 Jan;13(1):66-77. Curr Opin Drug Discov Devel. 2010. PMID: 20047147 Free PMC article. Review.
Cited by
-
Quantitative Prediction of Drug-Drug Interactions Involving Inhibitory Metabolites in Drug Development: How Can Physiologically Based Pharmacokinetic Modeling Help?CPT Pharmacometrics Syst Pharmacol. 2016 Oct;5(10):505-515. doi: 10.1002/psp4.12110. Epub 2016 Sep 19. CPT Pharmacometrics Syst Pharmacol. 2016. PMID: 27642087 Free PMC article.
-
Warfarin-amiodarone drug-drug interactions: determination of [I](u)/K(I,u) for amiodarone and its plasma metabolites.Clin Pharmacol Ther. 2012 Apr;91(4):709-17. doi: 10.1038/clpt.2011.283. Epub 2012 Mar 7. Clin Pharmacol Ther. 2012. PMID: 22398967 Free PMC article.
-
In vivo information-guided prediction approach for assessing the risks of drug-drug interactions associated with circulating inhibitory metabolites.Drug Metab Dispos. 2012 Aug;40(8):1487-94. doi: 10.1124/dmd.112.045799. Epub 2012 May 4. Drug Metab Dispos. 2012. PMID: 22563046 Free PMC article.
-
Inhibition of CYP2C19 and CYP3A4 by omeprazole metabolites and their contribution to drug-drug interactions.Drug Metab Dispos. 2013 Jul;41(7):1414-24. doi: 10.1124/dmd.113.051722. Epub 2013 Apr 25. Drug Metab Dispos. 2013. PMID: 23620487 Free PMC article.
-
Stereoselective inhibition of CYP2C19 and CYP3A4 by fluoxetine and its metabolite: implications for risk assessment of multiple time-dependent inhibitor systems.Drug Metab Dispos. 2013 Dec;41(12):2056-65. doi: 10.1124/dmd.113.052639. Epub 2013 Jun 19. Drug Metab Dispos. 2013. PMID: 23785064 Free PMC article.
References
-
- Zhang X, Jones DR, Hall SD. Prediction of the effect of erythromycin, diltiazem, and their metabolites, alone and in combination, on CYP3A4 inhibition. Drug Metab Dispos. 2009 Jan;37(1):150–160. - PubMed
-
- Zhang X, Quinney SK, Gorski JC, Jones DR, Hall SD. Semiphysiologically based pharmacokinetic models for the inhibition of midazolam clearance by diltiazem and its major metabolite. Drug Metab Dispos. 2009 Aug;37(8):1587–1597. - PubMed
-
- Isoherranen N, Kunze KL, Allen KE, Nelson WL, Thummel KE. Role of itraconazole metabolites in CYP3A4 inhibition. Drug Metab Dispos. 2004 Oct;32(10):1121–1131. - PubMed
-
- Stevens JC, Wrighton SA. Interaction of the enantiomers of fluoxetine and norfluoxetine with human liver cytochromes P450. J Pharmacol Exp Ther. 1993 Aug;266(2):964–971. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

