Designing artificial enzymes by intuition and computation
- PMID: 21124375
- PMCID: PMC3443871
- DOI: 10.1038/nchem.473
Designing artificial enzymes by intuition and computation
Abstract
The rational design of artificial enzymes, either by applying physico-chemical intuition of protein structure and function or with the aid of computational methods, is a promising area of research with the potential to tremendously impact medicine, industrial chemistry and energy production. Designed proteins also provide a powerful platform for dissecting enzyme mechanisms of natural systems. Artificial enzymes have come a long way from simple α-helical peptide catalysts to proteins that facilitate multistep chemical reactions designed by state-of-the-art computational methods. Looking forward, we examine strategies employed by natural enzymes that could be used to improve the speed and selectivity of artificial catalysts.
Figures
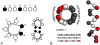

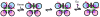
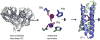
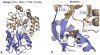

References
-
- Davie EAC, Mennen SM, Xu Y, Miller SJ. Asymmetric Catalysis Mediated by Synthetic Peptides. Chem Rev. 2007;107:5759–5812. - PubMed
-
- List B. Proline-catalyzed asymmetric reactions. Tetrahedron. 2002;58:5573–5590.
-
- Koder RL, Dutton PL. Intelligent design: the de novo engineering of proteins with specified functions. Dalton Transactions. 2006;25:3045–3051. - PubMed
-
- Lim V. Protein Folding. In: Jaenicke R, editor. 28th Conference of the German Biochemical Society; West Germany: Elservier, Regensburg; 1979. pp. 149–166.
-
- Crick FHC. The Packing of alpha-Helices: Simple Coiled Coils. Acta Crystallographica. 1953;6:689–697.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

