Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells
- PMID: 21124450
- PMCID: PMC3059717
- DOI: 10.1038/nature09571
Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells
Abstract
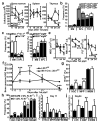
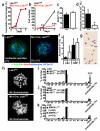
Little is known about metabolic regulation in stem cells and how this modulates tissue regeneration or tumour suppression. We studied the Lkb1 tumour suppressor and its substrate AMP-activated protein kinase (AMPK), kinases that coordinate metabolism with cell growth. Deletion of the Lkb1 (also called Stk11) gene in mice caused increased haematopoietic stem cell (HSC) division, rapid HSC depletion and pancytopenia. HSCs depended more acutely on Lkb1 for cell-cycle regulation and survival than many other haematopoietic cells. HSC depletion did not depend on mTOR activation or oxidative stress. Lkb1-deficient HSCs, but not myeloid progenitors, had reduced mitochondrial membrane potential and ATP levels. HSCs deficient for two catalytic α-subunits of AMPK (AMPK-deficient HSCs) showed similar changes in mitochondrial function but remained able to reconstitute irradiated mice. Lkb1-deficient HSCs, but not AMPK-deficient HSCs, exhibited defects in centrosomes and mitotic spindles in culture, and became aneuploid. Lkb1 is therefore required for HSC maintenance through AMPK-dependent and AMPK-independent mechanisms, revealing differences in metabolic and cell-cycle regulation between HSCs and some other haematopoietic progenitors.
Figures



Comment in
-
Stem cells: The blood balance.Nature. 2010 Dec 2;468(7324):644-5. doi: 10.1038/468644a. Nature. 2010. PMID: 21124447 No abstract available.
-
Stem cells: LKB1 maintains the balance.Nat Rev Mol Cell Biol. 2011 Jan;12(1):4. doi: 10.1038/nrm3032. Epub 2010 Dec 8. Nat Rev Mol Cell Biol. 2011. PMID: 21139635 No abstract available.
-
The tumor suppressor LKB1 emerges as a critical factor in hematopoietic stem cell biology.Cell Metab. 2011 Jan 5;13(1):8-10. doi: 10.1016/j.cmet.2010.12.015. Cell Metab. 2011. PMID: 21195344
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

