Structural changes of envelope proteins during alphavirus fusion
- PMID: 21124457
- PMCID: PMC3057476
- DOI: 10.1038/nature09546
Structural changes of envelope proteins during alphavirus fusion
Abstract
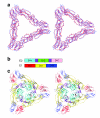
Alphaviruses are enveloped RNA viruses that have a diameter of about 700 Å and can be lethal human pathogens. Entry of virus into host cells by endocytosis is controlled by two envelope glycoproteins, E1 and E2. The E2-E1 heterodimers form 80 trimeric spikes on the icosahedral virus surface, 60 with quasi-three-fold symmetry and 20 coincident with the icosahedral three-fold axes arranged with T = 4 quasi-symmetry. The E1 glycoprotein has a hydrophobic fusion loop at one end and is responsible for membrane fusion. The E2 protein is responsible for receptor binding and protects the fusion loop at neutral pH. The lower pH in the endosome induces the virions to undergo an irreversible conformational change in which E2 and E1 dissociate and E1 forms homotrimers, triggering fusion of the viral membrane with the endosomal membrane and then releasing the viral genome into the cytoplasm. Here we report the structure of an alphavirus spike, crystallized at low pH, representing an intermediate in the fusion process and clarifying the maturation process. The trimer of E2-E1 in the crystal structure is similar to the spikes in the neutral pH virus except that the E2 middle region is disordered, exposing the fusion loop. The amino- and carboxy-terminal domains of E2 each form immunoglobulin-like folds, consistent with the receptor attachment properties of E2.
Figures

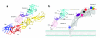

Comment in
-
Structural biology: An alphavirus puzzle solved.Nature. 2010 Dec 2;468(7324):645-6. doi: 10.1038/468645a. Nature. 2010. PMID: 21124448 Free PMC article.
Similar articles
-
Structural biology: An alphavirus puzzle solved.Nature. 2010 Dec 2;468(7324):645-6. doi: 10.1038/468645a. Nature. 2010. PMID: 21124448 Free PMC article.
-
An Alphavirus E2 Membrane-Proximal Domain Promotes Envelope Protein Lateral Interactions and Virus Budding.mBio. 2017 Nov 7;8(6):e01564-17. doi: 10.1128/mBio.01564-17. mBio. 2017. PMID: 29114027 Free PMC article.
-
Mapping the structure and function of the E1 and E2 glycoproteins in alphaviruses.Structure. 2006 Jan;14(1):63-73. doi: 10.1016/j.str.2005.07.025. Structure. 2006. PMID: 16407066 Free PMC article.
-
Cell entry of enveloped viruses.Adv Genet. 2011;73:121-83. doi: 10.1016/B978-0-12-380860-8.00004-5. Adv Genet. 2011. PMID: 21310296 Free PMC article. Review.
-
Mechanisms of coronavirus cell entry mediated by the viral spike protein.Viruses. 2012 Jun;4(6):1011-33. doi: 10.3390/v4061011. Epub 2012 Jun 20. Viruses. 2012. PMID: 22816037 Free PMC article. Review.
Cited by
-
Development of novel entry inhibitors targeting emerging viruses.Expert Rev Anti Infect Ther. 2012 Oct;10(10):1129-38. doi: 10.1586/eri.12.104. Expert Rev Anti Infect Ther. 2012. PMID: 23199399 Free PMC article. Review.
-
Development of a highly protective combination monoclonal antibody therapy against Chikungunya virus.PLoS Pathog. 2013;9(4):e1003312. doi: 10.1371/journal.ppat.1003312. Epub 2013 Apr 18. PLoS Pathog. 2013. PMID: 23637602 Free PMC article.
-
Seroprevalence of Getah virus in Pigs in Eastern China Determined with a Recombinant E2 Protein-Based Indirect ELISA.Viruses. 2022 Sep 30;14(10):2173. doi: 10.3390/v14102173. Viruses. 2022. PMID: 36298726 Free PMC article.
-
Interactions of the cytoplasmic domain of Sindbis virus E2 with nucleocapsid cores promote alphavirus budding.J Virol. 2012 Mar;86(5):2585-99. doi: 10.1128/JVI.05860-11. Epub 2011 Dec 21. J Virol. 2012. PMID: 22190727 Free PMC article.
-
An mRNA vaccine encoding Chikungunya virus E2-E1 protein elicits robust neutralizing antibody responses and CTL immune responses.Virol Sin. 2022 Apr;37(2):266-276. doi: 10.1016/j.virs.2022.01.032. Epub 2022 Jan 28. Virol Sin. 2022. PMID: 35527225 Free PMC article.
References
-
- Kielian M. Membrane fusion and the alphavirus life cycle. Adv. Virus Res. 1995;45:113–151. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

