Urine Peptidomic and Targeted Plasma Protein Analyses in the Diagnosis and Monitoring of Systemic Juvenile Idiopathic Arthritis
- PMID: 21124648
- PMCID: PMC2970804
- DOI: 10.1007/s12014-010-9058-8
Urine Peptidomic and Targeted Plasma Protein Analyses in the Diagnosis and Monitoring of Systemic Juvenile Idiopathic Arthritis
Abstract
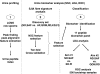
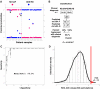
PURPOSE: Systemic juvenile idiopathic arthritis is a chronic pediatric disease. The initial clinical presentation can mimic other pediatric inflammatory conditions, which often leads to significant delays in diagnosis and appropriate therapy. SJIA biomarker development is an unmet diagnostic/prognostic need to prevent disease complications. EXPERIMENTAL DESIGN: We profiled the urine peptidome to analyze a set of 102 urine samples, from patients with SJIA, Kawasaki disease (KD), febrile illnesses (FI), and healthy controls. A set of 91 plasma samples, from SJIA flare and quiescent patients, were profiled using a customized antibody array against 43 proteins known to be involved in inflammatory and protein catabolic processes. RESULTS: We identified a 17-urine-peptide biomarker panel that could effectively discriminate SJIA patients at active, quiescent, and remission disease states, and patients with active SJIA from confounding conditions including KD and FI. Targeted sequencing of these peptides revealed that they fall into several tight clusters from seven different proteins, suggesting disease-specific proteolytic activities. The antibody array plasma profiling identified an SJIA plasma flare signature consisting of tissue inhibitor of metalloproteinase-1 (TIMP1), interleukin (IL)-18, regulated upon activation, normal T cell expressed and secreted (RANTES), P-Selectin, MMP9, and L-Selectin. CONCLUSIONS AND CLINICAL RELEVANCE: The urine peptidomic and plasma protein analyses have the potential to improve SJIA care and suggest that SJIA urine peptide biomarkers may be an outcome of inflammation-driven effects on catabolic pathways operating at multiple sites. ELECTRONIC SUPPLEMENTARY MATERIAL: The online version of this article (doi:10.1007/s12014-010-9058-8) contains supplementary material, which is available to authorized users.
Figures



References
-
- Cassidy JT, Levinson JE, Brewer EJ., Jr The development of classification criteria for children with juvenile rheumatoid arthritis. Bull Rheum Dis. 1989;38:1–7. - PubMed
-
- Sandborg C, Holmes TH, Lee T, et al. Candidate early predictors for progression to joint damage in systemic juvenile idiopathic arthritis. J Rheumatol. 2006;33:2322–2329. - PubMed
-
- Modesto C, Woo P, Garcia-Consuegra J, et al. Systemic onset juvenile chronic arthritis, polyarticular pattern and hip involvement as markers for a bad prognosis. Clin Exp Rheumatol. 2001;19:211–217. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
