Identification of a novel binding partner of phospholipase cβ1: translin-associated factor X
- PMID: 21124736
- PMCID: PMC2993962
- DOI: 10.1371/journal.pone.0015001
Identification of a novel binding partner of phospholipase cβ1: translin-associated factor X
Abstract
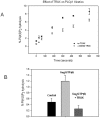
Mammalian phospholipase Cβ1 (PLCβ1) is activated by the ubiquitous Gα(q) family of G proteins on the surface of the inner leaflet of plasma membrane where it catalyzes the hydrolysis of phosphatidylinositol 4,5 bisphosphate. In general, PLCβ1 is mainly localized on the cytosolic plasma membrane surface, although a substantial fraction is also found in the cytosol and, under some conditions, in the nucleus. The factors that localize PLCβ1in these other compartments are unknown. Here, we identified a novel binding partner, translin-associated factor X (TRAX). TRAX is a cytosolic protein that can transit into the nucleus. In purified form, PLCβ1 binds strongly to TRAX with an affinity that is only ten-fold weaker than its affinity for its functional partner, Gα(q). In solution, TRAX has little effect on the membrane association or the catalytic activity of PLCβ1. However, TRAX directly competes with Gα(q) for PLCβ1 binding, and excess TRAX reverses Gα(q) activation of PLCβ1. In C6 glia cells, endogenous PLCβ1 and TRAX colocalize in the cytosol and the nucleus, but not on the plasma membrane where TRAX is absent. In Neuro2A cells expressing enhanced yellow and cyano fluorescent proteins (i.e., eYFP- PLCβ1 and eCFP-TRAX), Förster resonance energy transfer (FRET) is observed mostly in the cytosol and a small amount is seen in the nucleus. FRET does not occur at the plasma membrane where TRAX is not found. Our studies show that TRAX, localized in the cytosol and nucleus, competes with plasma-membrane bound Gα(q) for PLCβ1 binding thus stabilizing PLCβ1 in other cellular compartments.
Conflict of interest statement
Figures




References
-
- Suh P, Park J, Manzoli L, Cocco L, Peak J, et al. Multiple roles of phosphoinositide-specific phospholipase C isozymes. BMB reports. 2008;41:415–434. - PubMed
-
- Rebecchi MJ, Pentyala SN. Structure, function, and control of phosphoinositide-specific phospholipase C. PhysiolRev. 2000;80:1291–1335. - PubMed
-
- Exton JH. Regulation of phosphoinositide phospholipases by hormones, neurotransmitters, and other agonists linked to G proteins. Annual Review of Pharmacology & Toxicology. 1996;36:481–509. - PubMed
-
- Runnels LW, Jenco J, Morris A, Scarlata S. Membrane binding of phospholipases C-b1 and C-b2 is independent of phosphatidylinositol 4,5-bisphosphate and the a and bg subunits of G proteins. Biochemistry. 1996;35:16824–16832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

